An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List


A Comprehensive Review on Cannabis sativa Ethnobotany, Phytochemistry, Molecular Docking and Biological Activities
Sohaib hourfane, hicham mechqoq, abdellah yassine bekkali, joão miguel rocha, noureddine el aouad.
- Author information
- Article notes
- Copyright and License information
Correspondence: [email protected] (J.M.R.); [email protected] (N.E.A.); Tel.: +351-220-414-854 (J.M.R.); +212-661-186-156 (N.E.A.)
Received 2023 Feb 9; Revised 2023 Mar 3; Accepted 2023 Mar 7; Collection date 2023 Mar.
Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( https://creativecommons.org/licenses/by/4.0/ ).
For more than a century, Cannabis was considered a narcotic and has been banned by lawmakers all over the world. In recent years, interest in this plant has increased due to its therapeutic potential, in addition to a very interesting chemical composition, characterized by the presence of an atypical family of molecules known as phytocannabinoids. With this emerging interest, it is very important to take stock of what research has been conducted so far on the chemistry and biology of Cannabis sativa . The aim of this review is to describe the traditional uses, chemical composition and biological activities of different parts of this plant, as well as the molecular docking studies. Information was collected from electronic databases, namely SciFinder, ScienceDirect, PubMed and Web of Science. Cannabis is mainly popular for its recreational use, but it is also traditionally used as remedy for the treatment of several diseases, including diabetes, digestive, circulatory, genital, nervous, urinary, skin and respiratory diseases. These biological proprieties are mainly due to the presence of bioactive metabolites represented by more than 550 different molecules. Molecular docking simulations proved the presence of affinities between Cannabis compounds and several enzymes responsible for anti-inflammatory, antidiabetic, antiepileptic and anticancer activities. Several biological activities have been evaluated on the metabolites of Cannabis sativa , and these works have shown the presence of antioxidant, antibacterial, anticoagulant, antifungal, anti-aflatoxigenic, insecticidal, anti-inflammatory, anticancer, neuroprotective and dermocosmetic activities. This paper presents the up-to-date reported investigations and opens many reflections and further research perspectives.
Keywords: Cannabis sativa L., ethnobotany, chemical composition, biological activities, medicine, in silico, molecular docking
1. Introduction
Cannabis sativa L. is an herbaceous plant belonging to the Cannabaceae family. This plant species has many vernacular names and is known by many people as marijuana and hemp. Despite being native to Central Asia, this plant’s capacity of adaption to different climates lead to its spread all over the world [ 1 ]. The Cannabis genus is composed of a single specie named “ sativa ”, which regroup several subspecies or varieties including Cannabis sativa ssp. sativa , Cannabis sativa ssp. indica , Cannabis sativa ssp. ruderalis and Cannabis sativa ssp. afghanica . However, there is still controversy among the scientific community about the sub-classification of Cannabis species and varieties [ 2 , 3 , 4 ]. Cannabis sativa L. is one of the plants that have been used by humankind since antiquity, and many historians reported the different uses of this plant around the world [ 5 , 6 , 7 , 8 ]. The historical records show that this plant has been used as a source of fiber, food, oil, as well as for recreational and religious purposes. Additionally, several other uses have been developed through the centuries, such as livestock feed, skin and hair care [ 9 ]. Furthermore, many ethnobotanical surveys highlighted the therapeutic use of Cannabis sativa L. for the treatment of chronic pain, depression and inflammation. These activities have been justified by the original chemical composition, viz. Cannabis contains a large number of bioactive compounds with an estimation of more than 550 molecules [ 10 ]. Those compounds belong to the cannabinoid, terpenoid, stilbenoid, lignanamide, carotenoid, flavonoid and alkaloid classes [ 11 ]. The most notable compound of Cannabis remains the cannabinoids [ 12 , 13 ], a class of terpenolic compounds mainly found in the trichome cavity of female flowers [ 14 , 15 ]. Nowadays, Cannabis sativa L. is experiencing a renewed interest in many research fields, including microbiology and oncology [ 12 , 13 ]. In fact, the chemical diversity of cannabinoids proved to be very useful for targeting microorganisms such as bacteria, fungi, viruses, as well as cell components such as proteins and genes [ 16 ]. Additionally, their natural origin and low toxicity make them perfect candidates to treat hard-to-treat diseases, by solving therapeutic problems such as the resistance to antibiotics and, in the case of cancer treatment, the toxicity induced by ingestion and metabolization [ 17 , 18 , 19 ].
This review aims to present Cannabis sativa subspecies classification, description of the plant aspect and botany as well as a brief history and geographic distribution. This manuscript aims also to report and discuss the traditional uses as to both the preparation and administration modes of every part of the plant. It also aims to take into account the chemical composition of each part with the classification of the identified metabolites and their quantification, and the biological activities of Cannabis extracts and purified compounds. Finally, this review also includes the molecular docking studies of secondary metabolites previously identified in different parts of Cannabis sativa L.
2. Generalities about Cannabis sativa L.
2.1. plant nomenclature and synonyms.
Carolus Linnæus, also known as Carl von Linné (1707–1778), was the first person to frame principles for classification of living organisms into classes and sub-classes. His aim was to create a uniform international system for the identification of any living organism according to its morphological features. In this system, every organism is identified by his genera and specie names known as “binomial nomenclature”. In 1753, Carl von Linné mentioned the word Cannabis for the first time. This word comes from the Latin canna that means “reed” and bis that means “twice”, which means literally “reed with two sexes” [ 20 ]. Prior to the Linnæus nomenclature, Cannabis was widely used by different civilizations that gave it different names known as vernacular names [ 21 , 22 , 23 ]. At present, there are many local or vernacular names and various synonyms to name Cannabis. It is also known as hashish, marijuana, weed, Acapulco gold, ace, bat, bhang, log, hemp, Indian hemp, Colombian, doobie, dope (Cannabis), ganja, hydro, Jamaican, jive (sticks), joint, Maui wowie, Mexican, Panama gold, Panama red, pot, firecracker, ragweed, reefer, sativa, sinsemilla of California, spliff, Thai stick, etc. Those names and designations stay different depending on the region, country and culture. Cannabis sativa belongs to the Cannabaceae family, which includes 12 genera and 102 species, and with some species of economic importance, such as Humulus lupulus L. and Pteroceltis tatarinowii [ 24 ]. There are conflicting botanical classifications of Cannabis sativa , and the taxonomic classification of this plant has been the subject of divergences and debates. It is commonly accepted and recommended that Cannabis sativa is a single species [ 25 ], with four subspecies, namely indica , ruderalis, sativa and afghanica [ 2 , 3 , 4 , 26 ]. However, the classification criteria used for the differentiation of Cannabis sativa subspecies are often not very clear, since the chemical and morphological characteristics appear to vary according to the plant environment and pedology. In a study reported by Pacifico, et al. [ 27 ], the authors showed that the tetrahydrocannabinol (THC) content of a Cannabis sativa single species depends on the growing climate of the plant. In most cases, it is recommended to apply the name Cannabis sativa to all Cannabis plants encountered, since they all belong to the same species, and there is no agreement on the plant taxonomy [ 25 ].
2.2. Description and Botanical Aspect
Cannabis sativa L. is an annual, usually dioecious plant belonging to the Cannabaceae family [ 28 ]. It is now considered as the only species of the botanical genus Cannabis but divided into several phenotypes that can be described as subspecies or varieties [ 29 ]. Cannabis sativa has the particularity of being a fast-growing plant with a fluted stem that can reach 1 to 4 m with a diameter ranging between 1 and 3 cm ( Figure 1 a) [ 30 ]. The variation of height and diameter depends on the sub-species, environment, soil and climatic conditions [ 31 , 32 ]. The seeds are smooth, greyish ovoid or spherical in shape, 2.5 to 3.5 mm long and 2.5 to 3 mm in diameter ( Figure 1 c). Each seed contains two cotyledons rich in reserves (protein and oil), with an albumen considered particularly small compared to other plant species [ 33 ].

Cannabis sativa L. General aspect ( a ); inflorescence ( b ); seed ( c ); leaf ( d ); stem ( e ).
This plant is also characterized by long, fine flowers ( Figure 1 b). It has glandular hairs that make it fragrant and sticky [ 34 , 35 ]. At post-germination, young male and female plants cannot be distinguished. It is only during the last phase of growth, when flowers start appearing, that sex determination becomes possible [ 24 , 36 ]. The female flowers have no petals and consist of two long white, yellow or pink stigmas. Their calyx (less than 3–6 mm) envelops the ovary containing a single ovule. The female flowers appear in pairs in the axils of small leaves named bracts, these bracts contain numerous glandular trichomes where cannabinoids, mainly THC, accumulate [ 34 , 37 , 38 ]. On the other hand, the male flowers have five sepals of approximately 5 mm length, with yellow, white or green color [ 33 , 39 ]. The male plants develop small pollen sacs that serve to fertilize the female plants with hairy, resinous stigmas [ 34 , 36 , 40 ]. The Cannabis leaves are stipulate and opposite, with palmate (five to seven unequal), elongated and spiny segments with toothed margins ( Figure 1 d). Towards the top of the axis, the leaves become alternate and are inserted on the stem in an opposite arrangement every 10–30 cm [ 39 ]. These plants have cystolithic, tectorial and resin-secreting hairs; the latter have a voluminous base ending in a cluster of several cells, with each one secreting resin [ 39 ]. The root is taproot with a length of up to 30 cm, but the lateral roots reach 20 to 100 cm. In addition, in peaty soils, the lateral roots are more strongly developed, and the main root grows to a depth of 10–20 cm [ 41 ]. The growth rate of the root system is quite slow in the initial stages of vegetation, in contrast to the aerial part of the Cannabis plant, which grows intensively and rapidly [ 41 ].
2.3. Geographic Distribution and History
In nature, Cannabis is an annual flowering plant. This means that it completes its life cycle, from germination to seed production, in one year [ 42 ]. Cannabis can grow in a vast majority of climates ( Figure 2 ). From its region of origin, it appreciates calcareous and nitrogenous soils with a neutral or slightly acidic pH [ 43 , 44 ].
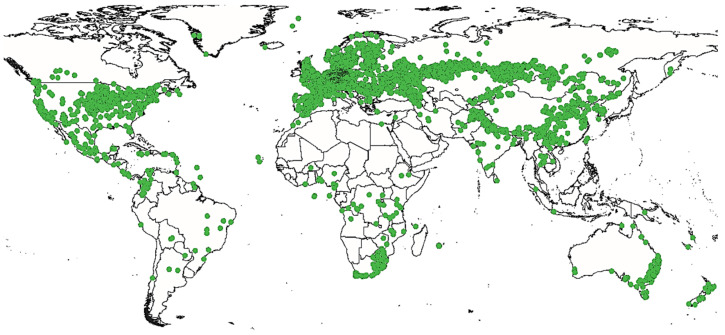
Geographic distribution of Cannabis sativa L.
This species originates from equatorial and subtropical regions, mainly from central Asia [ 1 ], where two places seem to be its cradle: the foothills of the Himalayas and the plains of the Pamir (a high mountain range centered in eastern Tajikistan with extensions into Afghanistan, the Republic of China and Kyrgyzstan) [ 45 ]. However, this plant has a wide geographical distribution growing up in Canada, United States of America, Europe and Africa. Cannabis is an ancient plant but the craze it has generated over (at least) the last century has greatly changed its face and even the face of the world. It is probably the first plant domesticated by humankind [ 46 ]. Many historical reports prove that this plant had been cultivated worldwide for thousands of years. The oldest documented evidence of Cannabis cultivation is a 26,900 B.C. hemp rope found in the Czech Republic [ 47 ]. Some of the earliest known prolific uses of Cannabis began in China around 10,000 B.C., where Cannabis was used to make clothing, rope and paper [ 48 ]. Further traces were reportedly found at the Neolithic site of Xianrendong on Chinese ceramics dating back to 8000 B.C. and decorated with hemp braided fibers. Between 8000 and 300 B.C., Cannabis was also cultivated in Japan and employed to make cloth fiber and paper [ 49 , 50 ]. However, the earliest reference of Cannabis psychotropic use goes back to 2700 B.C. It has been mentioned in the Chinese pharmacopoeia of the Emperor Chen Nong, where it is recommended as a sedative and remedy for insanity. Cannabis was also mentioned on the Ebers Papyrus of pharaonic Egypt back to 1550 B.C. as remedy for vaginal inflammations [ 51 ]. Yet, it was mentioned in Greek medicine, in the writings of Dioscorides, who underlines the psychotropic properties of the plant and already Galen fears that “it hurts the brain when we take too much” [ 52 , 53 ]. In India, it was one of the five magical plants used in religious rituals in the form of fumigation. In fact, around 1300 B.C., the stimulating and euphoric powers of bhanga (hemp in Sanskrit) were praised by the Indo-Aryans in one of the four holy books, the Atharva Veda [ 54 ]. Back to the European Continent, and around 700 B.C. in Marseille (France), Cannabis was used for rope manufacturing. The name Cannebiere (important avenue of the city) testifies of the importance of Cannabis at that time [ 35 ]. Jamestown settlers introduced Cannabis to colonial America in the early 1600s for the manufacture of rope, paper and other fiber products. This plant was so important that American presidents George Washington and Thomas Jefferson grew Cannabis [ 38 ]. The question of when and how Cannabis originated in the new world is still very controversial indeed. Cannabis was discovered in native American civilizations prior to Columbus’ arrival [ 55 ]. William Henry Holmes’ 1896 report “prehistoric textile art of the Eastern United States” indicated that Cannabis originated with native American tribes of the Great Lakes and Mississippi valley [ 56 ]. Cannabis products from pre-Columbian indigenous civilizations have also been found in Virginia [ 57 ]. Cannabis was an important crop in the United States until 1937, when the Marihuana Tax Act all but wiped out the American hemp industry. During World War II, Cannabis experienced a resurgence in the United States of America, as it was widely used to manufacture military items ranging from uniforms to canvas and rope [ 57 ]. At present, the most notable development in Cannabis production around the world is the rise of indoor cultivation, particularly in Europe, Australia and North America. This type of cultivation gives rise to a very lucrative trade, which is increasingly a source of profit for local organized crime groups [ 58 ].
3. Methodology
Relevant information about Cannabis sativa L. was collected from various scientific sources including SciFinder, ScienceDirect, PubMed and Web of Science. The targeted databases were probed with “Cannabis sativa”, “botany”, “history”, “ethnobotany”, “traditional use”, “phytochemistry”, “pharmacology”, “bioactivity”, “bioinformatic” and “in-silico prediction” as keywords. Thus, available articles were collected, summarized in tables and analyzed. In addition to that, we report up-to-date studies of Cannabis sativa ethnopharmacology, chemical composition, pharmacology and molecular docking simulations; this review aims to give a subjective critique to reported articles and offer perspectives for further investigations on Cannabis phytochemistry and pharmacology.
4. Results and Discussion
4.1. traditional uses of cannabis sativa l..
Cannabis sativa L. has been used in a wide variety of fields and showed a high usability potential with many applications including manufacturing of tools, construction, cosmetics, medication, shelter insulation, papermaking, human nutrition, animal feed, agrofuels, composite materials in association with plastics, etc. [ 5 , 6 , 7 , 8 ]. Table 1 summarizes the parts of the plant and their traditional uses.
Traditional uses of different parts of Cannabis sativa .
The analysis of collected data shows that seeds and leaves are the most used parts for medication. Many studies reported the use of Cannabis seed as food. It is used for the production of pasta, gluten-free flour characterized by a nutty taste, beer and oil [ 72 , 73 ]. In addition to their use for human nutrition [ 59 ], the seeds are also used to treat nausea, vomiting, stimulate the appetite of AIDS patients, cancer and hepatitis C. It is also applied as a muscle relaxant, for weight control, lung capacity enhancer and as an analgesic, anxiolytic, antiepileptic, antiemetic and against neurological pain [ 60 ]. These seeds have also a cosmetic use, mainly for hair fortification as a hair serum by external application of seed powder [ 61 ]. Beyond their potential value as medicine or food, Cannabis sativa seeds have recently been used to treat contaminated groundwater, since hemp seed protein powder proved to be more effective than other plant protein sources for chelating perfluoroalkyl and polyfluoroalkyl substances, known as “eternal chemicals” [ 74 ]. Those Cannabis proteins proved to be very useful for the treatment of salt-contaminated soils as well [ 75 ].
The leaves are externally used as poultice to treat eczema [ 61 ] and subcutaneous tissue disorders [ 62 ]. They are also orally consumed by local people for the treatment of central nervous system (CNS) disorders such as schizophrenia, gout, arthritic pain, bloating, coughing and mucus [ 63 , 64 ]. Cannabis leaves are among the most iconic symbols of modern stoner culture; their shape is frequently associated with the recreative use of this plant. Several studies described the traditional uses of Cannabis leaves, and those applications include the treatment of a wide range of health problems such as hypertension, rheumatoid arthritis, itching, cancer, snake and scorpion poisoning [ 66 ], as well as gastric and circulatory system disorders [ 62 ]. These leaves have also been described as strong analgesics, sedatives and narcotics [ 76 ]. Other studies described the use of Cannabis sativa stem fibers as firewood [ 62 ], for construction, tools, clothes, paper and rope manufacturing [ 5 , 59 ]. These fibers are obtained by a process called defibration, which can be briefly described as a stem beating and grinding. During this process, two co-products are obtained, namely chenevotte and Cannabis dust. It is important to highlight that the quality of the fiber decreases with the maturity of the plant, since the fibers become harder and coarser. In addition to the aerial parts of Cannabis plant, the root parts are also used for medication. They are used in particular for the treatment of joint pain, skin burns, inflammation, vermin and erysipelas infection [ 70 ]. These roots are also orally used in the form of juice to relieve issues stemming from childbirth, postpartum and hemorrhage [ 70 ]. Among the most cited uses of Cannabis sativa , the psychoactive remains the most present. The leaves and inflorescences have been consumed as a narcotic in different forms and have been prepared using different methods; for instance, the leaves are smoked or prepared, the inflorescences or resin are processed into charas or attar, hashish, ganja and plant powder, whereas leaves, inflorescences and shoots are used to prepare drinks (e.g., bhang, thandai, tandai, etc.) [ 5 , 77 ]. Moreover, Klauke, et al. [ 62 ] reported the religious use of Cannabis drinks. This plant preparation, referred to as traditional bhang drink, is highly consumed during Indian festivals such as Shivaratri and Holi [ 78 ]. Some ethnobotanical surveys described the used of the whole Cannabis plant. The aerial parts are mostly used for the treatment of mental disorders and nervous-system-related conditions. However, the most common use of those parts is for the treatment of gastric disorders, diabetes, scarring and asthma [ 71 ]. Conversely, some studies reported the appetite-stimulating, antidysentery and antidiarrhea effects of Cannabis inflorescences, but omitted the mention of preparation and administration modes [ 62 ]. The analysis of ethnobotanical findings shows that Cannabis sativa is a plant that was integrally exploited by local populations; some traditional uses are common to many countries whereas others are specific to some cultures. One can cite the psychotropic, medicinal and cosmetic purposes found all over the world, in contrast with the religious uses exclusively reported in Asian and Latin American countries. The previously reported activities and proprieties are mainly due to the presence of metabolites with interesting chemical structures; the ethnobotanical uses of Cannabis attracted phytochemists to investigate its chemical composition. The first compound to be identified and isolated from Cannabis was cannabinol at the end of the 19th century [ 79 ].
4.2. Chemical Composition of Cannabis sativa L.
Numerous studies have shown the importance of Cannabis secondary metabolites as well as their roles. This plant offers a rich reservoir of bioactive molecules that can be used for the production of pharmaceutical, nutraceutical and cosmetic products. Table 2 regroups the chemical composition of Cannabis sativa seeds, flowers, leaves and resin.
Chemical composition of Cannabis sativa different plant parts.
Cannabidiol (CBD); cannabidivarine (CBDV); cannabicitran (CBTC); cannabigerol (CBG); cannabichromene (CBC); cannabinol (CBN); δ9-tetrahydrocannabinol (δ 9 -THC); cannabidiolic acid (CBDA); tetrahydrocannabinolic acid (THCA); delta-8-tetrahydrocannabinol (δ 8 -THC); cannabigerol (CBG); cannabigerol acid (CBGA); cannabielsoin (CBE); cannabicitran (CBTC); cannabiripsol (CBR); total cannabidiol (tCBD); total cannabigerol (tCBG); total tetrahydrocannabinol (tTHC).
The chemical investigations conducted in different Cannabis sativa plant parts shows that terpenes, polyphenols and cannabinoids are the main represented secondary metabolites. Terpenes are represented by more than 100 molecules identified in the flowers, roots and leaves, as well as in the secretory glandular hairs considered as the main production site [ 11 , 98 ]. Furthermore, more than 20 polyphenols have been identified, and they are mainly flavonoids belonging to the flavone and flavonol subclasses [ 99 ]. Concerning the cannabinoids, they are among the most represented metabolites of Cannabis despite being represented by less than 20 molecules.
Cannabis seeds contain approximately 40% oil, 30% fibers and 25% proteins [ 85 , 100 ]. Those oils are rich in triacylglycerols (TAGs) represented by 18 different molecules; the predominating tags were LLL and OLLD with respective values of 23 and 19% [ 100 ]. Moreover, those oils contain high amounts of polyunsaturated fatty acids, which represent approximately 80% total fatty acids [ 101 ]. The fatty acid composition is characterized by the predominance of linoleic acid with range values of 45–60%, followed by oleic acid and palmitic with respective range values of 15–40% and 5–6% [ 80 , 81 ].
Hemp seeds contains also considerable amounts of polyphenols and tocopherols. According to Babiker, et al. [ 81 ], the hydroalcoholic extract of Cannabis seeds contained many polyphenols such as gallic acid (12.9 ± 18.3 mg/100 g) and catechin (6.0 ± 5.2 mg/100 g), whereas Moccia, et al. [ 83 ] reported the presence of additional polyphenols, namely quercetin-o-glucoside, n-trans-caffeoyltyramine and rutin. Moreover, another sub-class of polyphenols known as cannabisins have been reported on the seed hydroalcoholic maceration by Moccia, et al. [ 83 ]. The last authors described the presence of 11 molecules, namely cannabisin A, B, C, D, E, F, G, I, M, N and O, although these compounds have not been quantified. Concerning the tocopherols, four different isomers have been identified: the lead tocopherols are γ- and δ-tocopherols with 426 and 33 mg/kg, respectively [ 82 , 84 ].
Cannabinoids are a group of C21 or C22 terpenolic compounds mainly produced in Cannabis. They have also been reported in other plant species of the genus Radula and Helichrysum [ 102 ]. These cannabinoids are mainly present in leaves and inflorescences. However, Marzorati, et al. [ 103 ] reported the presence of some cannabinoids in the hydroalcoholic maceration of seeds, and Stambouli, et al. [ 104 ] reported the presence of mainly the cannabinoids tetrahydrocannabinol (THC), cannabidiol (CBD) and cannabigerol (CBG) in seed oil. These results are probably due to contamination of seeds by inflorescence resins, since cannabinoids are not produced or transported to the seeds [ 105 ]. Moreover, phytosterols, a group of lipids with a structure similar to cholesterols, mainly found in vegetable oils, have been identified in seed oils. Aiello, et al. [ 85 ] and Stambouli, et al. [ 84 ] mentioned the presence of this class of metabolites represented by the β-sitosterol, with 65–90%, and campesterol, with 6–17%. Furthermore, Stambouli, et al. [ 84 ] described the presence of an additional phytosterol known as δ-5-avenasterol, with a value of 7.8%. The carotenoids are among the less represented compounds in Cannabis seeds. They have been identified in the seed ethanolic extract, whereas lutein and β-carotene have been reported as major sterols, with 2.5 and 0.5 mg/100 g of dry weight, respectively [ 82 ].
The composition in hemp seeds of fatty acids, polyphenols, phytosterols, proteins and fibers, and precisely the presence of insoluble fibers in addition to a wide variety of minerals represented by phosphorus, potassium, magnesium, sulfur and calcium, as well as modest amounts of iron and zinc (an important enzyme cofactor for immunity and food absorption), widely justifies its biological proprieties and importance for human nutrition [ 106 , 107 ].
The Cannabis leaves contain terpenes, polyphenols, cannabinoids and alkaloids. The leaf essential oils are characterized by the presence of (e)-caryophyllene (28.3 ± 4.1%), α-humulene (9.3 ± 1.1%), β-selinene (4.7 ± 0.9%), caryophyllene oxide (4.3 ± 0.9%), α-selinene (3.1± 0.6%) and α-trans-bergamotene (2.7 ± 0.5%) [ 86 ]. These volatile terpenes are generally found in photosynthetic plant leaves and plays the role of protection from parasites and water loss [ 108 ]. The polyphenols of Cannabis leaves are mainly flavonoids and glycosides with apigenin and luteolin. These flavonoids represent 4 mg per g of the plant material. Elsewhere, the cannabinoids of Cannabis leaves had been described by Nagy, et al. [ 86 ]. The authors reported the presence of cannabidiol (CBD), cannabidivarine (CBDV), tetrahydrocannabinol (THC) and cannabichromene (CBC) with 11, 0.8, 0.7 and 0.5%, respectively. Moreover, Zagórska-Dziok, et al. [ 87 ] reported the lead presence of CBDA and CBD, with respective values of 150 and 31 mg/g of dry matter. Similarly to the cannabinoids, the alkaloids protect plants from predators and regulate plant growth [ 109 ]. These alkaloids have been described in cannabis leaves; Fasakin, et al. [ 88 ] described the presence of some alkaloids, with cannabisativine (410.30 μg/g), cannabimine C (376.12 μg/g) and anhydrocannabisativine (218.11 μg/g) as lead compounds.
The flowers show a chemical composition qualitatively similar to the leaves. They are mainly composed of terpenes, polyphenols and cannabinoids. The Cannabis flower terpenes have been identified in the essential oils obtained by hydrodistillation. According to Nagy, et al. [ 86 ], these essential oils are mainly composed of (E)-caryophyllene, α-humulene, β-selinene and α-selinene, with values of 29, 10, 4 and 3%, respectively. Additionally, Fischedick, et al. [ 110 ] reported the presence of mono- and sesquiterpenes in Cannabis flower essential oils. This study featured the quantification of monoterpenes and proved that they dominate the chemical composition, with a concentration of 28.3 mg/g of dry weight. These monoterpenes are represented by d-limonene, β-myrcene, α- and β-pinene. Furthermore, sesquiterpenes are represented by β-caryophyllene and α- humulene. These sesquiterpenes represent a concentration ranging between 0.5 and 10.1 mg/g of dry weight [ 110 ]. Polyphenols and cannabinoids have also been identified in the methanolic extract of flowers. The main polyphenols of flowers are quercetin di-c-hexoside and luteolin c-hexoside-2″-o-hexoside with 2.55 mg/g and 1.01 mg/g, respectively [ 86 ]. On the other hand, the cannabinoids are represented by cannabidiol (CBD), tetrahydrocannabinol (THC), cannabidivarine (CBDV), cannabicitran (CBTC) and cannabichromene (CBC), with respective values of 25, 1.5, 1.5, 1 and 0.2% [ 86 ]. The cannabinoidic composition of flowers fluctuates due to several environmental factors (e.g., temperature, soil nutrients, desiccation, insect predation and ultraviolet radiation) and genetic factors (varieties and heredity) [ 111 ]. According to Yang, et al. [ 112 ], the total THC, CBD and CBG increases significantly as the flowers matures, reaching the highest concentration during 6 to 7 weeks after anthesis.
The inflorescences of Cannabis have also been evaluated for their composition, and similar classes of metabolites have been described. These compounds belong to the same classes previously reported for the leaves and flowers. The essential oils of inflorescences have been reported in two research manuscripts. The first, published by Laznik, et al. [ 90 ], reported the presence of transcaryophyllene (38.2 ± 1.7%), nerolidol (12.7 ± 1.2%) and α-pinene (11.8 ± 0.4%), whereas the second, published by Pieracci et al. [ 91 ], reported the presence of β-caryophyllene (14.4 ± 0.89%), caryophyllene oxide (7.0 ± 1.06%) and α-humulene (5.3 ± 0.10%) as lead compounds. This variation is probably due to the variation of Cannabis sativa subspecies, cultivars and/or geographic, climatic and pedologic parameters. Laznik, et al. [ 90 ] also reported the chemical composition of inflorescence methanolic extract and concluded that this extract contained mainly cannabinoids represented by cannabidiolic acid (CBDA) and cannabidiol (CBD) with 9.5 and 8.7%, respectively.
Several studies have also described the composition of other Cannabis sativa L. parts, including roots, stem and resin. Sakakibara, et al. [ 113 ] and Lesma, et al. [ 114 ] reported the presence of phenolic amides and lignanamides in the fruits and roots of Cannabis. These compounds belong to the classes of lignans and polyphenols and are mainly cannabisin (A, B, C, D, E, F and G) and grossamide [ 99 ]. Triterpenes have also been found in Cannabis roots in form of friedelin and epifriedelanol [ 115 ]. The glandular secretory hairs are the main site of resin production. The latter is a yellow and sticky substance which contains the active principles. Some studies have shown its chemical composition. Stambouli, et al. [ 97 ] reported a high concentration of THC with a value higher than 20%. More recently, Elkins, et al. [ 96 ] identified and confirmed the high content of cannabinoids including cannabidiol (CBD) with a concentration of 72.12 μg/mL, followed by tetrahydrocannabinol (THC) with 48.02 μg/mL and cannabichromene (CBC) with 4.78 μg/mL. The concentrations of Cannabis sativa secondary metabolites depend on tissue type, age, variety, growing conditions (soil nutrition, temperature, humidity, UV radiation or light), harvest time (maturity) and storage conditions [ 80 , 111 , 116 , 117 ]. The Cannabis seeds are rich in oils and starch mainly composed of terpenes [ 118 ]. The seed oils serve as food for the young plant during the early stages of germination. In fact, the plant embryo needs a source of nutrition prior to its contact with soil and air [ 119 ]. Moreover, the leaves, flowers and inflorescences are rich in volatile terpenes, polyphenols and cannabinoids. These metabolites are generally involved in defense against ultraviolet radiation or aggression by pathogens. Unlike polyphenols, cannabinoids are more interesting due to their chemical structures and representativeness of Cannabis genus. The study of chemical composition of Cannabis plant parts is very important for the determination of biological activities. It is possible to predict a biological activity from the exhaustive chemical composition of an extract using bioinformatics tools such as molecular docking. This approach has been widely applied on Cannabis secondary metabolites, as described in the next section.
4.3. Molecular Docking Studies of Cannabis sativa L.
Molecular docking is a computational approach aiming to predict potential interactions between one or more ligands and a protein [ 120 ]. This approach predicts the optimal spatial conformation and orientation of the ligand within a protein active site, in addition to the determination of the interaction mode and binding affinity represented by a score. Molecular docking is the in silico equivalent of real high-throughput screening in which many molecules are tested against biological targets. The main goal of this approach is to discriminate active and inactive agents, in order to identify new molecules that will serve as a starting point for medicinal chemists [ 121 ].
The process of molecular docking can be subdivided into two basic steps, namely docking and scoring [ 122 ]. Docking is the step in which all possible spatial interactions between a ligand and a receptor are tested in order to identify the optimal interactions, whereas the binding affinity between the ligand and the receptor are quantified in scoring, and a score is given to the poses recorded after the docking phase.
Currently, there are more than 30 available docking software packages [ 123 ]. Most of them are also designed for virtual screening (independent dockings of multiple ligands with a protein). The three most frequently cited docking tools are AutoDock, GOLD and flex; they represent 27, 15 and 11% of the references, respectively [ 124 , 125 ].
Despite being very useful for guiding the selection of bioactive molecules for in vitro testing, the molecular docking simulations remains a prediction and can sometimes give erroneous results. They can be expressed either as a false negative, when an active molecule gives low docking affinity, or a false positive, when a non-active molecule is identified as a strong ligand [ 126 ]. However, this approach remains useful as a pre-investigation predictive tool. Concerning the Cannabis plant, several molecular docking studies have been reported on the different classes of metabolites, namely cannabinoids, terpenes, polyphenols, flavonoids, lignanamides, alkaloids, vitamins and proteins. These classes have been probed against a large number of specific enzymes that instigate important roles in different physiological processes (digestion, nerve conduction, hormone synthesis, etc.). Results of molecular docking are expressed in kcal/mol, and the lowest values correspond to higher affinity between a ligand and a protein. The studies reported in the bibliography are summarized in Table 3 .
Molecular docking of Cannabis sativa L. compounds.
Cannabichromene (CBC); cannabichromenic acid (CBCA); cannabichromevarin (CBCV); cannabichromevarinic acid (CBCVA); cannabicitran (CBTC); cannabicoumaronone (CBCN); cannabicyclol (CBL); cannabidiol (CBD); cannabidiol-c4 (CBDC4); cannabidiolic acid (CBDA); cannabidiorcol (CBDC); cannabidivarin (CBDV); cannabielsoin (CBE); cannabielsoin (CBL); cannabigerol (CBG); cannabigerolic acid (CBGA); cannabigerovarin (CBGV); cannabinodiol (CBND); cannabinodivarin (CBVD); cannabinol (CBN); cannabinol methyl ether (CBNM); cannabiripsol (CBR); cannabitriol (CBT); cannabivarin (CVN); tetrahydrocannabinol (THC); tetrahydrocannabivarin (THCV); δ-8-tetrahydrocannabinol (Δ-8-THC); δ-9-tetrahydrocannabinol (Δ-9-THC); δ9-tetrahydrocannabinolic acid (Δ-9-THCA).
4.3.1. Pesticidal Activity
Cholinesterase is an enzyme that catalyzes the hydrolysis reaction of a choline ester (acetylcholine, butyrylcholine) into choline and acetic acid. Acetylcholine is a well-known excitatory neurotransmitter that causes muscle contraction and stimulates the release of certain hormones. The inhibition of choline esterase causes the disfunction of nerve impulse transmission inducing mortality, and this activity is highly coveted for the elimination of pests and insects. The molecular docking of cannabis secondary metabolites against two types of cholinesterase, namely acetylcholinesterase (ACHE) and butyrylcholinesterase (BCHE), was described by Karimi, et al. [ 127 ]. In this article, the authors tested compounds belonging to the cannabinoid, flavonoid, terpene and phytosterol classes. For acetylcholinesterase, cannabioxepane, δ-9-THCA, Δ-8-THC and CBN showed scores lower than −10 kcal/mol, whereas cannabioxepane, CBL, CBN, CBT and Δ-8-THC showed scores lower than −8.5 kcal/mol against butyrylcholinesterase. Another investigation reported by Nasreen, et al. [ 128 ] reported the acetylcholinesterase docking with some cannabinoids, and CBD showed the best score with a value of −14.38 kcal/mol. Despite using the same docking software and acetylcholinesterase three-dimensional structure, the CBD score is better than the ones previously reported by Karimi, et al. [ 127 ]. This difference of results can be explained by the variation of docking parameters, such as the gridbox dimensions, position and the exhaustiveness.
4.3.2. Antimalarial and Anti-Leishmania Activities
The antimalarial activity of cannabinoids from Cannabis sativa was reported by Quan, et al. [ 129 ]. Their studied cannabinoids were docked against plasmodium falciparum dihydrofolate reductase-thymidinesynthase to recognize the potential binding affinities of these phytochemicals. Furthermore, the in silico antileishmanial activity of phytochemicals from Cannabis sativa has been well reported in the literature. In the studies conducted by Ogungbe, et al. [ 130 ], a molecular docking analysis was performed to examine the potential leishmania protein targets of plant-derived antiprotozoal polyphenolic compounds. A total of 352 phenolic phytochemicals—including 10 aurones, 6 cannabinoids, 34 chalcones, 20 chromenes, 52 coumarins, 92 flavonoids, 41 isoflavonoids, 52 lignans, 25 quinones, 8 stilbenoids, 9 xanthones and 3 miscellaneous phenolic compounds—were used in the virtual screening study with 24 leishmania enzymes (52 different protein structures from the protein data bank). Notable target proteins were leishmania dihydroorotate dehydrogenase, n-myristoyl transferase, phosphodiesterase b1, pteridine reductase, methionyl-trna synthetase, tyrosyl-trna synthetase, uridine diphosphate-glucose pyrophosphorylase, nicotinamidase and glycerol-3-phosphate dehydrogenase. The results showed that docked polyphenols can be considered as promising drug leads deserving further investigation.
4.3.3. Antiviral Activity
Despite great advances in medical and pharmaceutical research in recent years, diseases caused by viruses have remained a huge burden on public health like coronavirus, in particular SARS-CoV-2. In silico studies, including molecular docking, have repeatedly proved to be useful in addressing the particular challenges of antiviral drug discovery. A study published by Srivastava et al. [ 131 ] showed that cannabidiol may have an good affinity with a COVID-19 protease, and such affinity is represented by a score value of −7.10 kcal/mol. In another study, cannflavin exhibited a better score against an HIV-protease with a score of −9.70 kcal/mol [ 132 ].
4.3.4. Anti-Inflammatory Activity
Inflammation is a natural body reaction to injury and infection. It is mainly due to the deployment of immune system cells to the site of the injury or infection. The four symptoms of inflammation are heat, redness, swelling and pain. However, anti-inflammatory drugs are used to combat inflammation regardless of the cause of the inflammation. They are symptomatic treatments, i.e., they do not eliminate the cause of the inflammation but only its consequence and have an analgesic action. In a recent study, a panel of proteins, including the cellular tumor antigen p53, the essential modulator of NF-KB, the tumor necrosis factor (TNF) receptor, the transcription factor p65, NF-KB p105, the NF-k-b α inhibitor, the inhibitor of nuclear factor k-b kinase α subunit and the epidermal growth factor receptor, were identified as a primary target implicated in cannabidiol (CBD) anti-inflammatory activity. This finding was supported by molecular docking, which showed interactions between the major proteins and CBD. In addition, several signaling pathways, including TCF, toll-like receptors, mitogen-activated protein kinases, nuclear factor kappa, activated b-cell light chain activator and nucleotide-binding oligomerization domain receptors, were identified as key regulators in mediating the anti-inflammatory activity of CBD [ 136 ].
4.3.5. Anticancer Activity
Several molecular docking studies were interested in the potential anticancer activity of molecules derived from Cannabis sativa L. The molecular docking performed on placental aromatase cytochrome p450 was reported by Baroi, et al. [ 138 ]. These authors reported that cannabinoids, mainly cannabidiorcol and cannabidivarin, potentially bind with the best binding energies of −9.03 kcal/mol and −8.34 kcal/mol, respectively. In another study, molecular docking calculations were also performed to investigate the binding affinity of cannabinoids in the active site of crystal structure of the DLC1 RhoGAP domain in liver cancer 1. According to the performed calculations, cannabichromene and cannabidiolic acid showed promising results with regard to binding affinity to the target GTPase-activating proteins [ 137 ]. These compounds are held within the active site by a variety of non-covalent interactions, in particular hydrogen bonds, involving important amino acids. Similarly, cannabinoids were docked to predict their anti-inflammatory and anticancer activity. The researchers reported that cannabigerol and cannabichromene potentially bind to arachidonate 5-lypoxygenase with a binding energy score of −5.34 kcal/mol and −5.14 kcal/mol, respectively [ 142 ]. Cannabis flavonoids were also docked against topoisomerase II α to investigate the binding affinity of flavonoids in the active site of topoisomerase II α. The results showed that docked flavonoids can be considered as promising drug leads, thus deserving further investigation.
4.3.6. Antiepileptic Activity
A study published by Li, et al. [ 146 ] aimed to examine the mechanism of action of Cannabis on epilepsy, focusing on key compounds, targets and pathways. The molecular docking simulations were applied to identify the active ingredients and potential targets of Cannabis in the treatment of epilepsy. Topological analysis showed that cannabinoid receptor 1, albumin and glycogen synthase kinase-3 β (cnr1, alb and gsk3b) were the key targets with intense interaction. The results showed that cannabinol methyl ether could be the lead compound on the basis of molecular docking against docked protein targets. Therefore, these studies shed holistic light on the active components of Cannabis, which contributes to the search for lead compounds and the development of new drugs for the treatment of neurological diseases.
4.3.7. Neuroprotective Activity
Neuroprotective agents target the various deleterious mechanisms that occur in cerebral ischemia, with the aim of limiting the extension of the ischemic heart. It has been shown that the cannabinoid substances contained in the Cannabis sativa plant have great potential in a wide variety of therapeutic applications. However, its neuroprotective capacity has been the most studied in diseases such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, multiple sclerosis and amyotrophic lateral sclerosis [ 151 ]. The only example of proteins as infectious agents leading to neurodegenerative disorders was the prion protein. Since then, the characteristic self-seeding mechanism of the prion protein has also been attributed to other proteins associated with neurodegenerative diseases, notably amyloid-β (aβ). Modelling with the aβ monomer and pentamer revealed that cannabinoids interacted with the aβ protein mainly through steric interactions and hydrogen bonds. The results showed that CBG bound with the highest affinity of all the docked cannabinoids. The authors of this study reported that this was mainly due to the presence of a geranyl side chain in the CBG structure, as this side chain is associated with increased lipophilicity and may, therefore, increase the propensity to bind in the hydrophobic groove of the pentamer [ 152 ].
4.3.8. Dermocosmetic Activities
Dermocosmetic products are on the borderline between cosmetic products and medicines. Considered as cosmetic products, they are mainly used to ensure photoprotection of the skin, i.e., to limit the effects of its exposure to solar radiation, but also to improve the appearance of dry or aged skin, reduce inflammatory dermatological conditions (acne, couperose, seborrheic and atopic dermatitis, psoriasis, etc.), as well as for the care of nails and hair [ 153 ]. Furthermore, tyrosinase is a key enzyme in the process of melanogenesis (the biosynthesis of melanin). The dermocosmetic potential of Cannabis polyphenols has been reported, and the results showed a good affinity with the tyrosine phosphatase-1b with a free energy score of −24.34 kcal/mol [ 149 ]. Similarly, Cannabis alkaloids have been shown to possess affinity with tyrosinase with score a value of −3 kcal/mol [ 154 ].
4.4. Biological Activities of Cannabis sativa L.
Studies with Cannabis sativa L. have shown the presence of several biological activities, such as antioxidant, antibacterial, anticoagulant, insecticide, anticancer, anti-aflatoxigenic, antifungal, cytotoxic, anti-elastase, anti-collagenase, anti-acetylcholinesterase, anti-inflammatory, neuroprotective (anti-Alzheimer’s, anti-epilepsy and anti-Parkinson’s) and dermocosmetic (anti-tyrosinase, anti-collagenase and anti-elastase). Table 4 summarizes the biological activities of different Cannabis sativa parts according to the literature.
Biological activities of different parts of Cannabis sativa L.
IPC 50 : concentration providing 50% inhibition of lipid peroxidation/CC 50 : concentration providing 50% metal chelating activity. IC 50 : concentration of the sample that inhibits 50% expressed in mg/mL/ID: inhibition diameter (mm). EDTA: ethylenediaminetetraacetic acid./GAE: gallic acid equivalent./RE: rutin equivalent./CE: caffeic acid equivalent./TE: trolox equivalent. EDTAE: ethylenediaminetetraacetic acid equivalent./MIC: minimum inhibitory concentration./MBC: minimum bactericidal concentration. MCF-7: estrogen-dependent breast cancer cells./MDA-MB-468: triple-negative breast cancer cells./CACO-2: colorectal adenocarcinoma cells./MZ-CHA-1: cholangiocarcinoma cells.
4.4.1. Antioxidant Activity
An antioxidant is a molecule that slows down or prevents the oxidation of molecules that can play an important role in an organism metabolism. Cannabis sativa L. proved to have plenty of antioxidant substances. The antioxidant activity of this plant has been widely reported in the literature and it was determined by using many assays, including the free radical scavenging method (DPPH), oxygen radical absorbance capacity (ORAC), ferric reducing ability of plasma (FRAP) and 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) (ABTS), as well as other methods such as phosphomolybdenum and metal chelation. The antioxidant activity of Cannabis sativa has been reported in the plant seeds, leaves and aerial parts. Research on the antioxidant effects of Cannabis sativa L. seeds have been well reported in the literature. Manosroi, et al. [ 155 ] described the antioxidant activity of ethanolic extract using different tests, namely DPPH, chelating assay and lipid peroxidation inhibition. The obtained results showed that the seed organic extract exhibited strong antioxidant capacity, suggested by IC 50 (low inhibitory concentration at 50%) values with 14.5 mg/mL for DPPH, 1.9 mg/mL for chelating assay and 92.7 mg/mL for lipid peroxidation inhibition assay. In another study, the methanolic extract of the seeds showed an average activity against DPPH with an inhibition value of 75% at 500 µL/mL [ 83 ]. According to these authors, this activity is probably due to the presence of polyphenols and cannabinoids, known for their strong antioxidant capacity [ 164 , 165 ]. Moreover, other seed metabolites, such as lignamides [ 156 ] and proteins [ 157 ], exhibited average antioxidant activities. However, they are lower than the results obtained with polyphenols and cannabinoids. Considering the high amount of polyphenols in the leaves, the extracts obtained from these plant parts have generally shown strong antioxidant activity [ 87 , 155 ]. For example, the hydroalcoholic extract of the leaves showed a DPPH IC 50 value of 2.7 mg/mL [ 155 ]. This value is relatively lower than the result obtained in the seed DPPH assay, which suggests a higher antioxidant potential. Moreover, the aerial essential parts showed also excellent antioxidant activity, with values near to the positive control [ 163 ]. As previously mentioned, polyphenols are generally considered as the main group of antioxidant molecules that work through different mechanisms, such as the suppression of free radicals that initiate oxidative damage and inhibit the oxidation process, via chelation of catalytic metals or metal ions and the inhibition of lipoxygenase [ 166 , 167 ]. Furthermore, some volatile terpenes exhibits potent antioxidant and anti-free-radical properties [ 168 ]. This can explain the antioxidant activity of the essential oils of the aerial parts proven through different tests.
4.4.2. Antimicrobial Activity
An antimicrobial is a molecule with microbicidal (kills microorganisms) or microbiostatic (slow the microbial growth and/or development) activity. These substances have different names depending on the type of targeted microorganism, such as antibacterial (for bacteria), antifungal (for fungi), antiviral (for virus) or antiparasitic (for parasites). The antimicrobial activity of essential oils and organic extracts of different parts of Cannabis sativa L. against several microorganisms has been reported. However, the degree of antimicrobial activity varies from cultivar to cultivar [ 169 ], as well as according to the part of the plant used, the extraction method and type of extract. The seed hydroalcoholic extract was evaluated against Gram-positive and -negative bacteria, namely Staphylococcus aureus , Escherichia coli , Salmonella typhimurium , Enterobacter aerogenes , Enterococcus faecalis , Lacticaseibacillus paracasei , Limosilactobacillus reuteri , Levilactobacillus brevis , Lactiplantibacillus plantarum , Bifidobacterium bifidum , Bifidobacterium longum and Bifidobacterium breve , and the obtained results showed low antibacterial activity with MIC values superior to 1 mg/mL [ 158 ]. Regarding antibacterial tests on Cannabis leaves, a study published by Anjum [ 159 ] compared the efficacy of four extracts obtained with acetone, chloroform, ethanol and water against three bacterial strains, namely Escherichia coli , Staphylococcus aureus and Pseudomonas aeruginosa . The results showed similarities between the four extracts with values near to 19 mm. Moreover, the results published by Manosroi, et al. [ 155 ] showed the effect of ethanolic extract as an antibacterial agent against Staphylococcus mutans with an inhibition diameter of 1.33 ± 0.58 mm. The essential oils of the aerial parts were evaluated as well for their antibacterial activity. The volatile terpenes of Cannabis exerted diverse activity intensities according to the targeted bacterial strains, where the weakest antibacterial activity was observed against Helicobacter pylori and Klebsiella pneumonia strains, with MICs values of 64 and 38 mg/mL, respectively. Moreover, a weak antibacterial activity was observed against Micrococcus luteus and Staphylococcus aureus , with an MIC of 4.7 mg/mL for both strains, whereas a more moderate inhibitory activity was observed against Escherichia coli , Pseudomonas aeruginosa and Bacillus subtilis , with an MIC of 1.2 mg/mL [ 163 ]. These results are explained by the fact that volatile terpenes are known to be strong antibacterial compounds, according to many biological investigations [ 170 , 171 , 172 ].
Concerning the antifungal activity, Anjum [ 159 ] compared the efficacy of four Cannabis leaf extracts, obtained with acetone, chloroform, ethanol and water, against two fungi, namely Aspergillus niger and Fusarium spp. The extracts showed similar results with inhibition diameter values ranging between 20.6 and 23 mm for Aspergillus niger , and 18.3 to 24.3 mm for Fusarium spp. In another studies, the acetone extract of Cannabis flowers showed a significant effect on the growth of Aspergillus favus , the flower hydroalcoholic extract led to the inhibition of 36% of fungi mycelium at concentration of 7.2 mg of dry matter per mL of culture medium [ 161 ]. Moreover, aerial part essential oils showed interesting antifungal potential. Nafis, et al. [ 163 ] reported an MIC value of 9.5 mg/mL against four fungi species, namely Candida albicans , Candida glabrata , Candida krusei and Candida parapsilosis . However, Zengin, et al. [ 94 ] discovered a weak antifungal activity against a group of clinically relevant and multidrug-resistant microorganisms belonging to Candida spp. and Malassezia spp., with essential oil MIC values superior to 12.460 µg/mL.
4.4.3. Insecticidal Activity
Insecticides are active substances with the property of killing insects, their larvae and/or eggs. Insecticides act either by contact or after penetration into the digestive tract or into the respiratory system. Essential oils from inflorescences were evaluated for their mosquitocidal activities on larvae and pupae of two main malaria vectors known as Anopheles gambiae and Anopheles stephensi . The results showed that Cannabis inflorescence essential oils showed toxicity against mosquitoes with LC 50 values of 73.5 to 78.8 ppm for Anopheles stephensi larvae, and 20.13 to 67.19 ppm for pupae of Anopheles gambiae . Their natural origin and volatile propriety makes the use of these essential oils very attractive for the formulation of stable and safe biocontrol products [ 162 ]. The cholinesterase inhibition activity had also been reported in the literature. Furthermore, a molecule purified from the ethanolic extract of Cannabis seeds, namely 3,3′-demethyl-heliotropamide, showed a moderate activity with an IC 50 value of 46.2 µm [ 156 ], whereas the aerial part essential oils showed stronger activity against butyryl-choline esterase, with a concentration of 3.4 mg GALAE/g oil [ 94 ].
4.4.4. Anticoagulant Activity
It has been suggested that plants with anticoagulant activities act as herbal remedies that could lead to the discovery of new therapeutic agents to treat thrombosis-related diseases. Blood clotting studies were conducted to determine the possible antiprothrombotic effect of Cannabis leaf metabolites, with three main cannabinoids, THC, CBD and CBN, targeted. The in vitro effect of Cannabis extract on thrombin activity was evaluated by Coetzee, et al. [ 160 ]. In their publication, two cannabinoids, namely THC and CBN, showed interesting IC 50 values. The highest activity was obtained by THC, with a value of 1.79 mg/mL, whereas CBN showed weaker activity suggested by high IC 50 value. However, this study also featured an in vivo test applied on obese rats in order to determine the clotting times. As a result, the Cannabis-treated rats showed an efficiency of 50% with clotting two times higher than the control groups, thus proving that cannabinoids may have a good anticoagulant activity.
4.4.5. Antidiabetic Activity
Diabetes is a chronic, progressive and complex metabolic disorder characterized by abnormally high blood glucose levels. This condition is also known as hyperglycemia. Worldwide, approximately 90% of affected patients are non-insulin-dependent, classified as type 2 diabetes [ 173 ]. According to the World Health Organization (WHO), diabetes is a chronic disease that occurs when the pancreas does not produce enough insulin, or the body does not properly use the produced insulin. Insulin is a hormone that regulates the concentration of sugar in the blood. Antidiabetic activity is generally evaluated by the quantification of α-amylase inhibition [ 174 ]. This enzyme is generally produced in the pancreas and plays a key enzyme role in the increase in blood sugar by breaking down dietary carbohydrates, such as starch, into simple monosaccharides in the digestive system, followed by the further α-glucosidase degradation into glucose which, upon absorption, enters in the bloodstream. Therefore, inhibition of the enzymes α-amylase and α-glucosidase can suppress carbohydrate digestion, delay glucose absorption and, consequently, reduce blood glucose levels [ 175 ]. The study published by Zengin, et al. [ 94 ] proved that Cannabis aerial part essential oils exhibited antidiabetic properties against the α-glucosidase enzyme with a value of 3.77 mmol ACAE/g oil. This essential oil has also been evaluated against α-amylase but showed no significant result.
4.4.6. Anticancer Activity
Cancer remains a major cause of morbidity and mortality worldwide. It is currently treated using classical approaches such as surgery, chemotherapy and radiotherapy. The toxic side effects associated with chemotherapy and radiotherapy often lead to adverse health effects. This explains the huge need for new drugs, safer to use with less side effects. Experimentally, several Cannabis-derived compounds demonstrated conclusive efficacy in vitro and in vivo on a wide range of cancer cell lines, including breast [ 176 ], prostate [ 177 ], cervix [ 178 ], brain [ 179 ], colon [ 180 ] and leukemia/lymphoma [ 181 ]. A number of in vitro and in vivo studies have demonstrated the effects of phytocannabinoids on tumor progression. These studies suggest that specific cannabinoids such as Δ9-THC and CBD induce apoptosis and inhibit proliferation in various cancer cell lines at concentrations ranging between 5 and 65 µm [ 176 , 182 , 183 , 184 , 185 , 186 , 187 ]. Moreover, combination of certain phytocannabinoids improved the anticancer activity of Cannabis preparations; for example, Armstrong, et al. [ 182 ] revealed that the combination of CBD and Δ9 -THC exhibited a stronger melanoma cell mortality in comparison with Δ9-THC alone. In general, phytochemicals in the Cannabis plant, and especially cannabinoids, are non-selective in their functions and limited in their differential activity on cancer cells with normal cells. Therefore, researchers show interest in isolating bioactive phytochemicals from Cannabis with potent anticancer properties and generating lead compounds based on the natural backbone of a molecule as a synthetic approach.
4.4.7. Anti-Inflammatory and Analgesic Activities
Many compounds of Cannabis proved to have strong anti-inflammatory activity. The Cannabis seeds showed an inflammation-reducing capacity, especially on primary human monocytes treated with LPS. The results proved that the compounds of these seeds decreased the respective expression and secretion of IL-6 genes and TNF-α. Additionally, cannabinoids proved to be strong anti-inflammatory agents; in fact, they can suppress the production of pro-inflammatory cytokines and chemokines and may have therapeutic applications in health conditions underlying inflammatory components [ 188 , 189 ]. Analgesic action is defined as any procedure whose principle of activity is to reduce pain. This can be not only a drug but also any other method aimed at achieving analgesia, i.e., the abolition of the sensation of pain [ 190 ]. Clinical and experimental studies showed that Cannabis-derived compounds act as analgesic agents. However, the effectiveness of each product is variable and depends on the administration mode. With opioids being the only therapy for severe pain, the analgesic capacity of cannabinoids could provide a much-needed alternative to opioids [ 191 ]. Furthermore, cannabinoids act synergistically with opioids and act as opioid-sparing agents, allowing lower doses and fewer side effects of chronic opioid treatment [ 192 ]. Thus, the rational use of Cannabis-based medicines deserves to be seriously considered to alleviate patients’ suffering from severe pain.
4.4.8. Neuroprotective Activity
The terpenes and cannabinoids of Cannabis proved also to have neuroprotective proprieties. The neuroprotective effects of 17 compounds present in the aerial parts of Cannabis sativa L. were evaluated in PC-12 cells including p-hydroxybenzaldehyde, (e)-methyl p-hydroxycinnamate and ferulic acid—which showed additional protective effects against H 2 O 2 -induced damage [ 193 ]. Furthermore, di Giacomo, et al. [ 147 ] reported the neuroprotective and neuromodulatory effects induced by cannabidiol and cannabigerol in rat Hypo-E22 cells and isolated hypothalamus, whereas Landucci, et al. [ 194 ] proved that appropriate concentrations of CBD or CBD/THC ratios can represent a valid therapeutic intervention in the treatment of post-ischemic neuronal death. In another study, Esposito, et al. [ 195 ] highlighted the importance of CBD as a promising new drug able to reduce neuroinflammatory responses evoked by β-amyloid. Furthermore, the study published by Perez, et al. [ 196 ] described the neuronal counting of both motor and sensory neurons after CBD treatment using immunohistochemical analysis. The obtained results showed an increase by 30% of synaptic preservation on the spinal cord for the CBD-treated group, suggesting an average neuroprotective effect.
4.4.9. Antiepileptic and Anticonvulsant Activities
Despite being well-known for its psychoactive proprieties, Cannabis sativa L. has been investigated for additional effects on the central nervous system. A recently published study showed that CBD has a high efficacy in epilepsy with hippocampal focus than with the extrahippocampal amygdala and parvalbumin, implying a protective role in regulating hippocampal seizures and neurotoxicity at a juvenile age [ 197 ]. The efficiency of CBD has been further replicated in human populations, including adolescents and young adults with severe childhood-onset epilepsy [ 198 ]. In a 12-week open-label trial, a group of patients aged between 1 and 30 years were treated with 25 and 50 mg/kg of CBD. The treated groups showed a reduction in epileptic attack severity by 36.5%. Preclinical research has also attempted a more chronic elucidation of the efficacy of CBD as an anticonvulsant. Using the PTZ model of epilepsy, it was found that a reduction in seizure activity could be achieved with varying doses between 20 and 50 mg/kg of CBD over a 28-day treatment period [ 199 ]. Other cannabinoids, such as cannabigerol, cannabidivarin, cannabichromene, δ 9 -tetrahydrocannabinolic acid and tetrahydrocannabivarin, showed efficacy in models of Huntington’s disease and epilepsy. It is important to note that these phytocannabinoids and their combinations are warranted in a range of other neurodegenerative disorders such as Parkinson’s [ 200 ].
4.4.10. Dermocosmetic Activity
Lipids in human skin play a very important role in preserving the structure of the dermis, protecting it from dehydration. However, during menopause, hormonal changes negatively affect the skin’s balance, making it more prone to developing dryness [ 201 ]. The underlying tissues, such as subcutaneous adipose tissue and muscles, undergo atrophy due to the overproduction of some enzymes—among them tyrosinase, elastase and collagenase. Tyrosinase is mainly responsible for the production of melanin on the skin, whereas collagenase and elastase target the skin structural proteins collagen and elastin and degrade them, respectively. The results published by Manosroi, et al. [ 155 ] showed that leaf extracts have anti-tyrosinase activity with an IC 50 value of 0.07 ± 0.06 mg/mL, which suggest a strong tyrosinase inhibitory activity. Similarly, Zagórska-Dziok et al. [ 87 ] showed respective collagenase and elastase inhibitory activities of 80 and 30% at 1000 µg/mL. These activities are mainly due to the presence of polyphenols and cannabinoids.
4.5. Drugs Based on Cannabis sativa L.
Due to the importance of the biological evaluation’s findings, several Cannabis-based commercial pharmaceuticals have been produced. These products have many biological proprieties and were produced to treat a wide range of conditions. The first reported product has been commercialized under the name “marinol”. This drug was developed 40 years ago, in 1985, by an American pharmaceutical company, and used dronabinol and synthetic THC as active agents [ 202 ]. Likewise, “syndros” is another drug marketed in the USA in 2016 and it contains the same active ingredients [ 202 ]. Both drugs are indicated for the treatment of severe nausea and vomiting related to cancer chemotherapy and AIDS-anorexia associated with weight loss. Another active compound known as “nabilone” is used as ingredient of two drugs, “casamet” and “canemes” [ 203 ]. The “nabilone” is a synthetic analogue of THC approved by the U.S. Food and Drug Administration (U.S. FDA) for the treatment of chemotherapy and AIDS symptoms [ 204 ]. Two other cannabinoids, namely “CBD” and “THC”, are also used in two drugs commercialized under the name of Bourneville and used against two types of severe epilepsy (Lennox–Gastaut syndrome and Dravet syndrome) [ 205 ], whereas Sativex, generally known as “nabiximols”, is used to alleviate muscle spasms in multiple sclerosis disease.
5. Conclusions
This manuscript has reviewed and analyzed the historical, botanical, ethnopharmacological, chemical, bioinformatics and biological knowledge of Cannabis sativa from the earliest human communities to current medical applications, with a critical analysis of the multiple potential applications of cannabinoids in the contemporary scientific context.
At present, more than 545 phytochemicals have been described in the different parts of the Cannabis plant. The most represented metabolite class is the phytocannabinoids and they exhibit enormous structural diversity and bioactivities. Cannabis sativa is found in a wide variety of forms and environments on all continents and its pharmacological properties seem to go far beyond psychotic effects, with the ability to address needs such as the treatment and relief of many symptoms and diseases.
Furthermore, the relaxation of regulatory standards for therapeutic Cannabis and the conduct of more controlled clinical trials suggests that the Cannabis sativa plant has interesting therapeutic potential as an antiemetic, appetite stimulant in debilitating diseases (cancer and AIDS), analgesic, as well as in the treatment of multiple sclerosis, spinal cord injury, Tourette syndrome, epilepsy and glaucoma. Further clinical research is needed to investigate the potential therapeutic uses of this plant in specific medical conditions. Scientifically designed trials will help establish which of the cannabinoids produce the various beneficial effects described, or whether these are the result of a combination of cannabinoids. The research would also help to better characterize the adverse effects of each cannabinoid.
Acknowledgments
We would like to thank COST Action 18101 SOURDOMICS—Sourdough biotechnology network towards novel, healthier and sustainable food and bioprocesses ( https://sourdomics.com/ ; https://www.cost.eu/actions/CA18101/ , accessed on 7 February 2023), where the author N.E.A. is member of the working groups 3, 4 and 7, and the author J.M.R. is the Chair and Grant Holder Scientific Representative and is supported by COST (European Cooperation in Science and Technology) ( https://www.cost.eu/ , accessed on 7 February 2023). COST is a funding agency for research and innovation networks. Regarding the author J.M.R., he is financially supported by LA/P/0045/2020 (ALiCE) and UIDB/00511/2020-UIDP/00511/2020 (LEPABE) funded by national funds through FCT/MCTES (PIDDAC).
Author Contributions
Conceptualization: N.E.A. and S.H.; Data collection: S.H., A.Y.B. and H.M.; Writing—original draft preparation: S.H. and H.M.; Writing—review and editing: H.M., J.M.R. and N.E.A.; Supervision: N.E.A. and J.M.R.; Funding acquisition: N.E.A. and J.M.R. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Data availability statement.
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
“Agence National des Plantes Médicinales et Aromatiques-Taounate, ANPMA” Morocco and University Abdelmalek Essaadi-Tetouan Morocco (VPMA4).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
- 1. McPartland J.M., Hegman W., Long T. Cannabis in Asia: Its center of origin and early cultivation, based on a synthesis of subfossil pollen and archaeobotanical studies. Veg. Hist. Archaeobot. 2019;28:691–702. doi: 10.1007/s00334-019-00731-8. [ DOI ] [ Google Scholar ]
- 2. Anderson L.C. Leaf variation among Cannabis species from a controlled garden. Bot. Mus. Leafl. Harv. Univ. 1980;28:61–69. doi: 10.5962/p.168641. [ DOI ] [ Google Scholar ]
- 3. McPartland J.M. Cannabis systematics at the levels of family, genus, and species. Cannabis Cannabinoid Res. 2018;3:203–212. doi: 10.1089/can.2018.0039. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 4. McPartland J.M., Small E. A classification of endangered high-THC cannabis (Cannabis sativa subsp. indica) domesticates and their wild relatives. PhytoKeys. 2020;144:81. doi: 10.3897/phytokeys.144.46700. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 5. Clarke R., Merlin M. Cannabis: Evolution and Ethnobotany. University of California Press; Berkeley, CA, USA: 2016. [ Google Scholar ]
- 6. Gedik G., Avinc O. Sustainability in the Textile and Apparel Industries. Springer; Berlin/Heidelberg, Germany: 2020. Hemp fiber as a sustainable raw material source for textile industry: Can we use its potential for more eco-friendly production? pp. 87–109. [ Google Scholar ]
- 7. Gomez F.P., Hu J., Clarke M.A. Cannabis as a Feedstock for the Production of Chemicals, Fuels, and Materials: A Review of Relevant Studies To Date. Energy Fuels. 2021;35:5538–5557. doi: 10.1021/acs.energyfuels.0c04121. [ DOI ] [ Google Scholar ]
- 8. Rupasinghe H.V., Davis A., Kumar S.K., Murray B., Zheljazkov V.D. Industrial hemp (Cannabis sativa subsp. sativa) as an emerging source for value-added functional food ingredients and nutraceuticals. Molecules. 2020;25:4078. doi: 10.3390/molecules25184078. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 9. Fleming M.P., Clarke R.C. Physical evidence for the antiquity of Cannabis sativa L. J. Int. Hemp Assoc. 1998;5:80–95. [ Google Scholar ]
- 10. Lowe H., Steele B., Bryant J., Toyang N., Ngwa W. Non-cannabinoid metabolites of Cannabis sativa L. with therapeutic potential. Plants. 2021;10:400. doi: 10.3390/plants10020400. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 11. Andre C.M., Hausman J.-F., Guerriero G. Cannabis sativa: The plant of the thousand and one molecules. Front. Plant Sci. 2016;7:19. doi: 10.3389/fpls.2016.00019. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 12. Giupponi L., Leoni V., Carrer M., Ceciliani G., Sala S., Panseri S., Pavlovic R., Giorgi A. Overview on Italian hemp production chain, related productive and commercial activities and legislative framework. Ital. J. Agron. 2020;15:194–205. doi: 10.4081/ija.2020.1552. [ DOI ] [ Google Scholar ]
- 13. Pollastro F., Minassi A., Fresu L.G. Cannabis phenolics and their bioactivities. Curr. Med. Chem. 2018;25:1160–1185. doi: 10.2174/0929867324666170810164636. [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Taura F., Sirikantaramas S., Shoyama Y., Shoyama Y., Morimoto S. Phytocannabinoids in Cannabis sativa: Recent studies on biosynthetic enzymes. Chem. Biodivers. 2007;4:1649–1663. doi: 10.1002/cbdv.200790145. [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Brenneisen R. Marijuana and the Cannabinoids. Humana Press; Clifton, NJ, USA: 2007. Chemistry and analysis of phytocannabinoids and other Cannabis constituents; pp. 17–49. [ Google Scholar ]
- 16. Whiting P.F., Wolff R.F., Deshpande S., Di Nisio M., Duffy S., Hernandez A.V., Keurentjes J.C., Lang S., Misso K., Ryder S. Cannabinoids for medical use: A systematic review and meta-analysis. Jama. 2015;313:2456–2473. doi: 10.1001/jama.2015.6358. [ DOI ] [ PubMed ] [ Google Scholar ]
- 17. Mansoori B., Mohammadi A., Davudian S., Shirjang S., Baradaran B. The different mechanisms of cancer drug resistance: A brief review. Adv. Pharm. Bull. 2017;7:339. doi: 10.15171/apb.2017.041. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 18. Zaman S.B., Hussain M.A., Nye R., Mehta V., Mamun K.T., Hossain N. A review on antibiotic resistance: Alarm bells are ringing. Cureus. 2017;9:e1403. doi: 10.7759/cureus.1403. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 19. Kennedy L.B., Salama A.K. A review of cancer immunotherapy toxicity. CA A Cancer J. Clin. 2020;70:86–104. doi: 10.3322/caac.21596. [ DOI ] [ PubMed ] [ Google Scholar ]
- 20. Pollio A. The name of Cannabis: A short guide for nonbotanists. Cannabis Cannabinoid Res. 2016;1:234–238. doi: 10.1089/can.2016.0027. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 21. Bruneau D. Ph.D. Thesis. University of Rennes; Rennes, France: 2016. Le Cannabis sativa: Une plante psychotrope ayant des intérêtsthérapeutiques. [ Google Scholar ]
- 22. Russo E.B. History of cannabis and its preparations in saga, science, and sobriquet. Chem. Biodivers. 2007;4:1614–1648. doi: 10.1002/cbdv.200790144. [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. Wujastyk D. Cannabis in Traditional Indian Herbal Medicine. Ayurveda at the Crossroads of Care and Cure, Centro de Historia del Alêmm-Mar, Universidade Nova de Lisboa; Lisbon, Portugal: 2002. pp. 45–73. [ Google Scholar ]
- 24. Hazekamp A. Cannabis Review. Volume 2009 Department of Plant Metobolomics, Leiden University; Leiden, The Netherlands: 2008. [ Google Scholar ]
- 25. Farag S., Kayser O. Handbook of Cannabis and Related Pathologies. Elsevier; Amsterdam, The Netherlands: 2017. The cannabis plant: Botanical aspects; pp. 3–12. [ Google Scholar ]
- 26. Schultes R.E., Klein W.M., Plowman T., Lockwood T.E. Cannabis: An example of taxonomic neglect. In: Rubin V., editor. Cannabis and Culture. De Gruyter Mouton; Berlin, NY, USA: 1975. pp. 21–38. [ Google Scholar ]
- 27. Pacifico D., Miselli F., Carboni A., Moschella A., Mandolino G. Time course of cannabinoid accumulation and chemotype development during the growth of Cannabis sativa L. Euphytica. 2008;160:231–240. doi: 10.1007/s10681-007-9543-y. [ DOI ] [ Google Scholar ]
- 28. Small E., Cronquist A. A practical and natural taxonomy for Cannabis. Taxon. 1976;25:405–435. doi: 10.2307/1220524. [ DOI ] [ Google Scholar ]
- 29. Clarke R.C., Merlin M.D., Small E. Evolution and classification of Cannabis sativa (Marijuana, Hemp) in relation to human utilization. Bot. Rev. 2015;81:189–294. [ Google Scholar ]
- 30. Amaducci S., Zatta A., Raffanini M., Venturi G. Characterisation of hemp (Cannabis sativa L.) roots under different growing conditions. Plant Soil. 2008;313:227–235. doi: 10.1007/s11104-008-9695-0. [ DOI ] [ Google Scholar ]
- 31. Amaducci S., Zatta A., Pelatti F., Venturi G. Influence of agronomic factors on yield and quality of hemp (Cannabis sativa L.) fibre and implication for an innovative production system. Field Crops Res. 2008;107:161–169. doi: 10.1016/j.fcr.2008.02.002. [ DOI ] [ Google Scholar ]
- 32. Campiglia E., Radicetti E., Mancinelli R. Plant density and nitrogen fertilization affect agronomic performance of industrial hemp (Cannabis sativa L.) in Mediterranean environment. Ind. Crops Prod. 2017;100:246–254. doi: 10.1016/j.indcrop.2017.02.022. [ DOI ] [ Google Scholar ]
- 33. Bouloc P. Le Chanvre Industriel: Production et Utilisations. France Agricole Editions; Paris, France: 2006. [ Google Scholar ]
- 34. Anwar F., Latif S., Ashraf M. Analytical characterization of hemp (Cannabis sativa) seed oil from different agro-ecological zones of Pakistan. J. Am. Oil Chem. Soc. 2006;83:323–329. doi: 10.1007/s11746-006-1207-x. [ DOI ] [ Google Scholar ]
- 35. Bouloc P. Hemp: Industrial Production and Uses. CABI; Wallingford, UK: 2013. [ Google Scholar ]
- 36. Schilling S., Melzer R., McCabe P.F. Cannabis sativa. Curr. Biol. 2020;30:R8–R9. doi: 10.1016/j.cub.2019.10.039. [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Lynch R.C., Vergara D., Tittes S., White K., Schwartz C., Gibbs M.J., Ruthenburg T.C., DeCesare K., Land D.P., Kane N.C. Genomic and chemical diversity in Cannabis. Crit. Rev. Plant Sci. 2016;35:349–363. doi: 10.1080/07352689.2016.1265363. [ DOI ] [ Google Scholar ]
- 38. Newton D.E. Marijuana: A Reference Handbook. Abc-Clio; Santa Barbara, CA, USA: 2013. [ Google Scholar ]
- 39. Botineau M. Botanique Systématique et Appliquée des Plantes à Fleurs. Tec & doc; Paris, France: 2010. [ Google Scholar ]
- 40. Richard D., Senon J.-L. Le Cannabis. Presses universitaires de France; Paris, France: 2010. [ Google Scholar ]
- 41. Strzelczyk M., Lochynska M., Chudy M. Systematics and botanical characteristics of industrial hemp Cannabis sativa L. J. Nat. Fibers. 2021;19:5804–5826. doi: 10.1080/15440478.2021.1889443. [ DOI ] [ Google Scholar ]
- 42. Radosevich S.R., Holt J.S., Ghersa C. Weed Ecology: Implications for Management. John Wiley & Sons; Hoboken, NJ, USA: 1997. [ Google Scholar ]
- 43. Citterio S., Santagostino A., Fumagalli P., Prato N., Ranalli P., Sgorbati S. Heavy metal tolerance and accumulation of Cd, Cr and Ni by Cannabis sativa L. Plant Soil. 2003;256:243–252. doi: 10.1023/A:1026113905129. [ DOI ] [ Google Scholar ]
- 44. Magnusson K., Svennerstedt B. Influence of temperature on the water retting process of hemp (Cannabis sativa L.) cultivated under Swedish climate conditions. J. Ind. Hemp. 2007;12:3–17. doi: 10.1300/J237v12n02_02. [ DOI ] [ Google Scholar ]
- 45. Matthieu M.L. Les Cannabinoïdes Dans La Prise en Charge des Patients Sous Anticancereux et Antiretroviraux: Connaissances Actuelles et Perspectives D’avenir en France. Faculty of Pharmacy, University of Lille; Lille, France: 2015. [ Google Scholar ]
- 46. Li H.-L. An archaeological and historical account of cannabis in China. Econ. Bot. 1974;28:437–448. doi: 10.1007/BF02862859. [ DOI ] [ Google Scholar ]
- 47. Hill B. Legalized Marijuana: Canada Comes Round to the Wisdom of Ages, Ancient-Origins. [(accessed on 6 March 2023)]. Available online: https://www.ancient-origins.net/history/cannabis-journey-through-ages-003084 .
- 48. Abel E.L. Marihuana: The First Twelve Thousand Years. Springer Science & Business Media; Berlin, Germany: 2013. [ Google Scholar ]
- 49. Ramamoorthy S.K., Skrifvars M., Persson A. A review of natural fibers used in biocomposites: Plant, animal and regenerated cellulose fibers. Polym. Rev. 2015;55:107–162. doi: 10.1080/15583724.2014.971124. [ DOI ] [ Google Scholar ]
- 50. Turner C.E., Elsohly M.A., Boeren E.G. Constituents of Cannabis sativa L. XVII. A review of the natural constituents. J. Nat. Prod. 1980;43:169–234. doi: 10.1021/np50008a001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 51. Veiga P. Oncology and Infectious Diseases in Ancient Egypt: The Ebers Papyrus’ Treatise on Tumours 857–877 and the Cases Found in Ancient Egyptian Human Material. University of Manchester; Manchester, UK: 2009. [ Google Scholar ]
- 52. Dawson W.R. Studies in the Egyptian Medical Texts—III. J. Egypt. Archaeol. 1934;20:41–46. doi: 10.1177/030751333402000108. [ DOI ] [ Google Scholar ]
- 53. Faulkner R.O. The Ancient Egyptian Pyramid Texts. Aris & Phillips; Wiltshire, England: 1969. [ Google Scholar ]
- 54. Ferrara M.S. Peak-experience and the entheogenic use of cannabis in world religions. J. Psychedelic Stud. 2021;4:179–191. doi: 10.1556/2054.2020.00122. [ DOI ] [ Google Scholar ]
- 55. Gately I. Tobacco: A Cultural History of How an Exotic Plant Seduced Civilization. Open Road and Grove Atlantic; New York, NY, USA: 2007. [ Google Scholar ]
- 56. Holmes W.H. Prehistoric Textile Art of Eastern United States. Volume 13. Independently published; Washington, DC, USA: 1896. p. 44. [ Google Scholar ]
- 57. Deitch R. Hemp: American History Revisited: The Plant with a Divided History. Algora Publishing; New York, NY, USA: 2003. [ Google Scholar ]
- 58. Chouvy P.-A. Cannabis cultivation in the world: Heritages, trends and challenges. EchoGéo. 2019;48:21. doi: 10.4000/echogeo.17591. [ DOI ] [ Google Scholar ]
- 59. Liu F.-H., Hu H.-R., Du G.-H., Deng G., Yang Y. Ethnobotanical research on origin, cultivation, distribution and utilization of hemp (Cannabis sativa L.) in China. Indian J. Tradit. Knowl. 2017;16:235–242. [ Google Scholar ]
- 60. Singh R., Upadhyay S.K., Rani A., Kumar P., Sharma P., Sharma I., Singh C., Chauhan N., Kumar M. Ethnobotanical study of weed flora at district Ambala, Haryana, India: Comprehensive medicinal and pharmacological aspects of plant resources. Int. J. Pharm. Res. 2020;12:1941–1956. [ Google Scholar ]
- 61. El Khomsi M., Dandani Y., Chaachouay N., Hmouni D. Ethnobotanical study of plants used for medicinal, cosmetic, and food purposes in the region of Moulay Yacoub, Northeast of Morocco. J. Pharm. Pharmacogn. Res. 2022;10:13–29. doi: 10.56499/jppres21.1084_10.1.13. [ DOI ] [ Google Scholar ]
- 62. Klauke A.L., Racz I., Pradier B., Markert A., Zimmer A.M., Gertsch J., Zimmer A. The cannabinoid CB2 receptor-selective phytocannabinoid beta-caryophyllene exerts analgesic effects in mouse models of inflammatory and neuropathic pain. Eur. Neuropsychopharmacol. 2014;24:608–620. doi: 10.1016/j.euroneuro.2013.10.008. [ DOI ] [ PubMed ] [ Google Scholar ]
- 63. Rahmatullah M., Mollik M.A.H., Azam A., Islam M.R., Chowdhury M.A.M., Jahan R., Chowdhury M.H., Rahman T. Ethnobotanical survey of the Santal tribe residing in Thakurgaon District, Bangladesh. Am. Eurasian J. Sustain. Agric. 2009;3:889–898. [ Google Scholar ]
- 64. Mawla F., Khatoon S., Rehana F., Jahan S., Shelley M.M.R., Hossain S., Haq W.M., Rahman S., Debnath K., Rahmatullah M. Ethnomedicinal plants of folk medicinal practitioners in four villages of Natore and Rajshahi districts, Bangladesh. Am. Eur. J. Sustain. Agric. 2012;6:406–416. [ Google Scholar ]
- 65. Ahmed M., Azam K., Nur M. Traditional knowledge and formulations of medicinal plants used by the traditional medical practitioners of Bangladesh to treat schizophrenia like psychosis. Schizophr. Res. Treat. 2014;2014:679810. doi: 10.1155/2014/679810. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 66. Rahmatullah M., Mollik M.A.H., Khatun M.A., Jahan R., Chowdhury A.R., Seraj S., Hossain M.S., Nasrin D., Khatun Z. A survey on the use of medicinal plants by folk medicinal practitioners in five villages of Boalia sub-district, Rajshahi district, Bangladesh. Adv. Nat. Appl. Sci. 2010;4:39–44. [ Google Scholar ]
- 67. Benkhnigue O., Zidane L., Fadli M., Elyacoubi H., Rochdi A., Douira A. Etude ethnobotanique des plantes médicinales dans la région de Mechraâ Bel Ksiri (Région du Gharb du Maroc) Acta Botánica Barcinonensia. 2010;53:191–216. [ Google Scholar ]
- 68. Kona S., Rahman A. Inventory of medicinal plants at Mahadebpur upazila of Naogaon district, Bangladesh. Appl. Ecol. Environ. Sci. 2016;4:75–83. [ Google Scholar ]
- 69. Rahmatullah M., Azam M.N.K., Rahman M.M., Seraj S., Mahal M.J., Mou S.M., Nasrin D., Khatun Z., Islam F., Chowdhury M.H. A survey of medicinal plants used by Garo and non-Garo traditional medicinal practitioners in two villages of Tangail district, Bangladesh. Am. Eurasian J. Sustain. Agric. 2011;5:350–357. [ Google Scholar ]
- 70. Ryz N.R., Remillard D.J., Russo E.B. Cannabis roots: A traditional therapy with future potential for treating inflammation and pain. Cannabis Cannabinoid Res. 2017;2:210–216. doi: 10.1089/can.2017.0028. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 71. Bouarfa M., Lebtar S., Boukhira S., Bousta D. An ethnobotanical and ethnopharmacological survey of Cannabis sativa of Taounate Region in Northern Morocco. Int. J. Pharm. Sci. Rev. Res. 2020;64:116–122. doi: 10.47583/ijpsrr.2020.v64i02.019. [ DOI ] [ Google Scholar ]
- 72. Callaway J.C. Hempseed as a nutritional resource: An overview. Euphytica. 2004;140:65–72. doi: 10.1007/s10681-004-4811-6. [ DOI ] [ Google Scholar ]
- 73. Carus M., Sarmento L. The European Hemp Industry: Cultivation, processing and applications for fibres, shivs, seeds and flowers. Eur. Ind. Hemp Assoc. 2016;5:1–9. [ Google Scholar ]
- 74. Turner B.D., Sloan S.W., Currell G.R. Novel remediation of per-and polyfluoroalkyl substances (PFASs) from contaminated groundwater using Cannabis sativa L.(hemp) protein powder. Chemosphere. 2019;229:22–31. doi: 10.1016/j.chemosphere.2019.04.139. [ DOI ] [ PubMed ] [ Google Scholar ]
- 75. Cosarca S., Rosca I., Coman A., Bota M.C.N., Tanase C. Effect of treatment with saline solution (NaCl) on rape plants in presence of the hemp shives. Rev. Chim. 2017;68:1843–1846. doi: 10.37358/RC.17.8.5777. [ DOI ] [ Google Scholar ]
- 76. Hussain W., Ullah M., Dastagir G., Badshah L. Quantitative ethnobotanical appraisal of medicinal plants used by inhabitants of lower Kurram, Kurram agency, Pakistan. Avicenna J. Phytomed. 2018;8:313. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 77. Knapp A.A., Lee D.C., Borodovsky J.T., Auty S.G., Gabrielli J., Budney A.J. Emerging trends in cannabis administration among adolescent cannabis users. J. Adolesc. Health. 2019;64:487–493. doi: 10.1016/j.jadohealth.2018.07.012. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 78. Chopra I., Chopra R.N. The use of the cannabis drugs in India. Bull. Narc. 1957;9:4–29. [ Google Scholar ]
- 79. Wood T.B., Spivey W.N., Easterfield T.H. III.—Cannabinol. Part I. J. Chem. Soc. Trans. 1899;75:20–36. doi: 10.1039/CT8997500020. [ DOI ] [ Google Scholar ]
- 80. Ross S.A., ElSohly H.N., ElKashoury E.A., ElSohly M.A. Fatty acids of cannabis seeds. Phytochem. Anal. 1996;7:279–283. doi: 10.1002/(SICI)1099-1565(199611)7:6<279::AID-PCA322>3.0.CO;2-P. [ DOI ] [ Google Scholar ]
- 81. Babiker E.E., Uslu N., Al Juhaimi F., Ahmed I.A.M., Ghafoor K., Özcan M.M., Almusallam I.A. Effect of roasting on antioxidative properties, polyphenol profile and fatty acids composition of hemp (Cannabis sativa L.) seeds. LWT. 2021;139:110537. doi: 10.1016/j.lwt.2020.110537. [ DOI ] [ Google Scholar ]
- 82. Irakli M., Tsaliki E., Kalivas A., Kleisiaris F., Sarrou E., Cook C.M. Effect οf genotype and growing year on the nutritional, phytochemical, and antioxidant properties of industrial hemp (Cannabis sativa L.) seeds. Antioxidants. 2019;8:491. doi: 10.3390/antiox8100491. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 83. Moccia S., Siano F., Russo G.L., Volpe M.G., La Cara F., Pacifico S., Piccolella S., Picariello G. Antiproliferative and antioxidant effect of polar hemp extracts (Cannabis sativa L., Fedora cv.) in human colorectal cell lines. Int. J. Food Sci. Nutr. 2020;71:410–423. doi: 10.1080/09637486.2019.1666804. [ DOI ] [ PubMed ] [ Google Scholar ]
- 84. Stambouli H., El Bouri A., Bouayoun T., Bellimam A. Caractérisation de l’huile de graines de Cannabis sativa L. cultivé au nord du Maroc. Ann. Toxicol. Anal. 2006;18:119–125. doi: 10.1051/ata:2006004. [ DOI ] [ Google Scholar ]
- 85. Aiello A., Pizzolongo F., Scognamiglio G., Romano A., Masi P., Romano R. Effects of supercritical and liquid carbon dioxide extraction on hemp (Cannabis sativa L.) seed oil. Int. J. Food Sci. Technol. 2020;55:2472–2480. doi: 10.1111/ijfs.14498. [ DOI ] [ Google Scholar ]
- 86. Nagy D.U., Cianfaglione K., Maggi F., Sut S., Dall’Acqua S. Chemical characterization of leaves, male and female flowers from spontaneous Cannabis (Cannabis sativa L.) growing in Hungary. Chem. Biodivers. 2019;16:e1800562. doi: 10.1002/cbdv.201800562. [ DOI ] [ PubMed ] [ Google Scholar ]
- 87. Zagórska-Dziok M., Bujak T., Ziemlewska A., Nizioł-Łukaszewska Z. Positive effect of Cannabis sativa L. herb extracts on skin cells and assessment of cannabinoid-based hydrogels properties. Molecules. 2021;26:802. doi: 10.3390/molecules26040802. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 88. Fasakin O.W., Oboh G., Ademosun A.O., Lawal A.O. The modulatory effects of alkaloid extracts of Cannabis sativa, Datura stramonium, Nicotiana tabacum and male Carica papaya on neurotransmitter, neurotrophic and neuroinflammatory systems linked to anxiety and depression. Inflammopharmacology. 2022;30:2447–2476. doi: 10.1007/s10787-022-01006-x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 89. Guo T., Liu Q., Hou P., Li F., Guo S., Song W., Zhang H., Liu X., Zhang S., Zhang J. Stilbenoids and cannabinoids from the leaves of Cannabis sativa f. sativa with potential reverse cholesterol transport activity. Food Funct. 2018;9:6608–6617. doi: 10.1039/C8FO01896K. [ DOI ] [ PubMed ] [ Google Scholar ]
- 90. Laznik Ž., Košir I.J., Košmelj K., Murovec J., Jagodič A., Trdan S., Ačko D.K., Flajšman M. Effect of Cannabis sativa L. root, leaf and inflorescence ethanol extracts on the chemotrophic response of entomopathogenic nematodes. Plant Soil. 2020;455:367–379. doi: 10.1007/s11104-020-04693-z. [ DOI ] [ Google Scholar ]
- 91. Pieracci Y., Ascrizzi R., Terreni V., Pistelli L., Flamini G., Bassolino L., Fulvio F., Montanari M., Paris R. Essential Oil of Cannabis sativa L: Comparison of Yield and Chemical Composition of 11 Hemp Genotypes. Molecules. 2021;26:4080. doi: 10.3390/molecules26134080. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 92. Gunjević V., Grillo G., Carnaroglio D., Binello A., Barge A., Cravotto G. Selective recovery of terpenes, polyphenols and cannabinoids from Cannabis sativa L. inflorescences under microwaves. Ind. Crops Prod. 2021;162:113247. doi: 10.1016/j.indcrop.2021.113247. [ DOI ] [ Google Scholar ]
- 93. Vanhoenacker G., Van Rompaey P., De Keukeleire D., Sandra P. Chemotaxonomic features associated with flavonoids of cannabinoid-free cannabis (Cannabis sativa subsp. sativa L.) in relation to hops (Humulus lupulus L.) Nat. Prod. Lett. 2010;16:57–63. doi: 10.1080/1057563029001/4863. [ DOI ] [ PubMed ] [ Google Scholar ]
- 94. Zengin G., Menghini L., Di Sotto A., Mancinelli R., Sisto F., Carradori S., Cesa S., Fraschetti C., Filippi A., Angiolella L. Chromatographic analyses, in vitro biological activities, and cytotoxicity of Cannabis sativa L. essential oil: A multidisciplinary study. Molecules. 2018;23:3266. doi: 10.3390/molecules23123266. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 95. Palmieri S., Pellegrini M., Ricci A., Compagnone D., Lo Sterzo C. Chemical composition and antioxidant activity of thyme, hemp and coriander extracts: A comparison study of maceration, Soxhlet, UAE and RSLDE techniques. Foods. 2020;9:1221. doi: 10.3390/foods9091221. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 96. Elkins A.C., Deseo M.A., Rochfort S., Ezernieks V., Spangenberg G. Development of a validated method for the qualitative and quantitative analysis of cannabinoids in plant biomass and medicinal cannabis resin extracts obtained by super-critical fluid extraction. J. Chromatogr. B. 2019;1109:76–83. doi: 10.1016/j.jchromb.2019.01.027. [ DOI ] [ PubMed ] [ Google Scholar ]
- 97. Stambouli H., El Bouri A., Bouayoun T. Évolution de la teneur en Δ9-THC dans les saisies de résines de cannabis au Maroc de 2005 à 2014. Toxicol. Anal. et Clin. 2016;28:146–152. doi: 10.1016/j.toxac.2015.11.001. [ DOI ] [ Google Scholar ]
- 98. ElSohly M.A. Marijuana and the Cannabinoids. Springer Science & Business Media; Berlin, Germany: 2007. [ Google Scholar ]
- 99. Flores-Sanchez I.J., Verpoorte R. Secondary metabolism in cannabis. Phytochem. Rev. 2008;7:615–639. doi: 10.1007/s11101-008-9094-4. [ DOI ] [ Google Scholar ]
- 100. Taaifi Y., Benmoumen A., Belhaj K., Aazza S., Abid M., Azeroual E., Elamrani A., Mansouri F., Serghini Caid H. Seed composition of non-industrial hemp (Cannabis sativa L.) varieties from four regions in northern Morocco. Int. J. Food Sci. Technol. 2021;56:5931–5947. doi: 10.1111/ijfs.15136. [ DOI ] [ Google Scholar ]
- 101. Da Porto C., Decorti D., Tubaro F. Fatty acid composition and oxidation stability of hemp (Cannabis sativa L.) seed oil extracted by supercritical carbon dioxide. Ind. Crops Prod. 2012;36:401–404. doi: 10.1016/j.indcrop.2011.09.015. [ DOI ] [ Google Scholar ]
- 102. Appendino G., Gibbons S., Giana A., Pagani A., Grassi G., Stavri M., Smith E., Rahman M.M. Antibacterial cannabinoids from Cannabis sativa: A structure− activity study. J. Nat. Prod. 2008;71:1427–1430. doi: 10.1021/np8002673. [ DOI ] [ PubMed ] [ Google Scholar ]
- 103. Marzorati S., Friscione D., Picchi E., Verotta L. Cannabidiol from inflorescences of Cannabis sativa L.: Green extraction and purification processes. Ind. Crops Prod. 2020;155:112816. doi: 10.1016/j.indcrop.2020.112816. [ DOI ] [ Google Scholar ]
- 104. Stambouli H., El Bouri A., Bouayoun T., El Karni N., Naciri Z., Johar A., Saoura A., Saidi S. Expérimentation de la culture de chanvre industriel à fibres au Maroc. Ann. Toxicol. Anal. 2011;23:15–20. doi: 10.1051/ata/2011105. [ DOI ] [ Google Scholar ]
- 105. Jang E., Kim H., Jang S., Lee J., Baeck S., In S., Kim E., Kim Y.-U., Han E. Concentrations of THC, CBD, and CBN in commercial hemp seeds and hempseed oil sold in Korea. Forensic Sci. Int. 2020;306:110064. doi: 10.1016/j.forsciint.2019.110064. [ DOI ] [ PubMed ] [ Google Scholar ]
- 106. Deferne J.-L., Pate D.W. Hemp seed oil: A source of valuable essential fatty acids. J. Int. Hemp. Assoc. 1996;3:1–7. [ Google Scholar ]
- 107. Orhan İ., Küsmenoǧlu Ş., Şener B. GC-MS analysis of the seed oil of Cannabis sativa L. cultivated in Turkey. Gazi Univ. Eczaci. Fak. Derg. 2000;17:79–81. [ Google Scholar ]
- 108. Cunningham S.A., Summerhayes B., Westoby M. Evolutionary divergences in leaf structure and chemistry, comparing rainfall and soil nutrient gradients. Ecol. Monogr. 1999;69:569–588. doi: 10.1890/0012-9615(1999)069[0569:EDILSA]2.0.CO;2. [ DOI ] [ Google Scholar ]
- 109. Chik S.C., Or T.C., Luo D., Yang C.L., Lau A.S. Pharmacological effects of active compounds on neurodegenerative disease with gastrodia and uncaria decoction, a commonly used poststroke decoction. Sci. World J. 2013;2013:896873. doi: 10.1155/2013/896873. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 110. Fischedick J.T., Hazekamp A., Erkelens T., Choi Y.H., Verpoorte R. Metabolic fingerprinting of Cannabis sativa L., cannabinoids and terpenoids for chemotaxonomic and drug standardization purposes. Phytochemistry. 2010;71:2058–2073. doi: 10.1016/j.phytochem.2010.10.001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 111. Pate D.W. Chemical ecology of Cannabis. J. Int. Hemp. Assoc. 1994;2:32–37. [ Google Scholar ]
- 112. Yang R., Berthold E.C., McCurdy C.R., da Silva Benevenute S., Brym Z.T., Freeman J.H. Development of cannabinoids in flowers of industrial hemp (Cannabis sativa L.): A pilot study. J. Agric. Food Chem. 2020;68:6058–6064. doi: 10.1021/acs.jafc.0c01211. [ DOI ] [ PubMed ] [ Google Scholar ]
- 113. Sakakibara I., Ikeya Y., Hayashi K., Mitsuhashi H. Three phenyldihydronaphthalene lignanamides from fruits of Cannabis sativa. Phytochemistry. 1992;31:3219–3223. doi: 10.1016/0031-9422(92)83479-I. [ DOI ] [ Google Scholar ]
- 114. Lesma G., Consonni R., Gambaro V., Remuzzi C., Roda G., Silvani A., Vece V., Visconti G. Cannabinoid-free Cannabis sativa L. grown in the Po valley: Evaluation of fatty acid profile, antioxidant capacity and metabolic content. Nat. Prod. Res. 2014;28:1801–1807. doi: 10.1080/14786419.2014.926354. [ DOI ] [ PubMed ] [ Google Scholar ]
- 115. Slatkin D.J., Doorenbos N.J., Harris L.S., Masoud A.N., Quimby M.W., Schiff P.L. Chemical constituents of Cannabis sativa L. root. J. Pharm. Sci. 1971;60:1891–1892. doi: 10.1002/jps.2600601232. [ DOI ] [ PubMed ] [ Google Scholar ]
- 116. Keller A., Leupin M., Mediavilla V., Wintermantel E. Influence of the growth stage of industrial hemp on chemical and physical properties of the fibres. Ind. Crops Prod. 2001;13:35–48. doi: 10.1016/S0926-6690(00)00051-0. [ DOI ] [ Google Scholar ]
- 117. Kushima H., Shoyama Y., Nishioka I. Cannabis. Xii. Variations of cannabinoid contents in several strains of Cannabis sativa L. with leaf-age, season and sex. Chem. Pharm. Bull. 1980;28:594–598. doi: 10.1248/cpb.28.594. [ DOI ] [ Google Scholar ]
- 118. Mechqoq H., El Yaagoubi M., Momchilova S., Msanda F., El Aouad N. Comparative study on yields and quality parameters of argan oils extracted by conventional and green extraction techniques. Grain Oil Sci. Technol. 2021;4:125–130. doi: 10.1016/j.gaost.2021.08.002. [ DOI ] [ Google Scholar ]
- 119. Copeland L.O., McDonald M.F. Principles of Seed Science and Technology. Springer Science & Business Media; Berlin, Germany: 2012. [ Google Scholar ]
- 120. Morris G.M., Lim-Wilby M. Molecular Modeling of Proteins. Springer; Berlin/Heidelberg, Germany: 2008. Molecular docking; pp. 365–382. [ DOI ] [ PubMed ] [ Google Scholar ]
- 121. Hourfane S., Mechqoq H., Errajouani F., Rocha J.M., El Aouad N. In Vitro and In Silico Evaluations of Boswellia carterii Resin Dermocosmetic Activities. Cosmetics. 2022;9:131. doi: 10.3390/cosmetics9060131. [ DOI ] [ Google Scholar ]
- 122. Kitchen D.B., Decornez H., Furr J.R., Bajorath J. Docking and scoring in virtual screening for drug discovery: Methods and applications. Nat. Rev. Drug Discov. 2004;3:935–949. doi: 10.1038/nrd1549. [ DOI ] [ PubMed ] [ Google Scholar ]
- 123. Sousa S.F., Fernandes P.A., Ramos M.J. Protein–ligand docking: Current status and future challenges. Proteins Struct. Funct. Bioinform. 2006;65:15–26. doi: 10.1002/prot.21082. [ DOI ] [ PubMed ] [ Google Scholar ]
- 124. Chen G., Seukep A.J., Guo M. Recent advances in molecular docking for the research and discovery of potential marine drugs. Mar. Drugs. 2020;18:545. doi: 10.3390/md18110545. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 125. Grosdidier A., Zoete V., Michielin O. EADock: Docking of small molecules into protein active sites with a multiobjective evolutionary optimization. Proteins Struct. Funct. Bioinform. 2007;67:1010–1025. doi: 10.1002/prot.21367. [ DOI ] [ PubMed ] [ Google Scholar ]
- 126. Muegge I., Rarey M. Small molecule docking and scoring. Rev. Comput. Chem. 2001;17:1–60. [ Google Scholar ]
- 127. Karimi I., Yousofvand N., Hussein B.A. In vitro cholinesterase inhibitory action of Cannabis sativa L. Cannabaceae and in silico study of its selected phytocompounds. Silico Pharmacol. 2021;9:1–15. doi: 10.1007/s40203-021-00075-0. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 128. Nasreen N., Niaz S., Khan A., Zaman M.A., Ayaz S., Naeem H., Khan N., Elgorban A.M. The potential of Allium sativum and Cannabis sativa extracts for anti-tick activities against Rhipicephalus (Boophilus) microplus. Exp. Appl. Acarol. 2020;82:281–294. doi: 10.1007/s10493-020-00540-z. [ DOI ] [ PubMed ] [ Google Scholar ]
- 129. Quan P.M., Toan T.Q., Hung N.P., Nam P.H., Kiet N.T., Ha N.X., Le D.T.T., An T.N.T., Show P.L., Thi H.H.P. Cannabis sativa L. chemical compositions as potential plasmodium falciparum dihydrofolate reductase-thymidinesynthase enzyme inhibitors: An in silico study for drug development. Open Chem. 2021;19:1244–1250. doi: 10.1515/chem-2021-0102. [ DOI ] [ Google Scholar ]
- 130. Ogungbe I.V., Erwin W.R., Setzer W.N. Antileishmanial phytochemical phenolics: Molecular docking to potential protein targets. J. Mol. Graph. Model. 2014;48:105–117. doi: 10.1016/j.jmgm.2013.12.010. [ DOI ] [ PubMed ] [ Google Scholar ]
- 131. Srivastava A.K., Kumar A., Misra N. On the inhibition of COVID-19 protease by Indian herbal plants: An in silico investigation. arXiv. 20202004.03411 [ Google Scholar ]
- 132. Akhtar A., Hussain W., Rasool N. Probing the pharmacological binding properties, and reactivity of selective phytochemicals as potential HIV-1 protease inhibitors. Univ. Sci. 2019;24:441–464. doi: 10.11144/Javeriana.SC24-3.artf. [ DOI ] [ Google Scholar ]
- 133. Nouadi B., Ezaouine A., El Messal M., Blaghen M., Bennis F., Chegdani F. Prediction of anti-COVID 19 therapeutic power of medicinal Moroccan plants using molecular docking. Bioinform. Biol. Insights. 2021;15:11779322211009199. doi: 10.1177/11779322211009199. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 134. Ngo S.T., Quynh Anh Pham N., Thi Le L., Pham D.-H., Vu V.V. Computational determination of potential inhibitors of SARS-CoV-2 main protease. J. Chem. Inf. Model. 2020;60:5771–5780. doi: 10.1021/acs.jcim.0c00491. [ DOI ] [ PubMed ] [ Google Scholar ]
- 135. Tallei T.E., Tumilaar S.G., Niode N.J., Kepel B.J., Idroes R., Effendi Y., Sakib S.A., Emran T.B. Potential of plant bioactive compounds as SARS-CoV-2 main protease (Mpro) and spike (S) glycoprotein inhibitors: A molecular docking study. Scientifica. 2020;2020:6307457. doi: 10.1155/2020/6307457. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 136. Ma H., Xu F., Liu C., Seeram N.P. A Network Pharmacology Approach to Identify Potential Molecular Targets for Cannabidiol’s Anti-Inflammatory Activity. Cannabis Cannabinoid Res. 2021;6:288–299. doi: 10.1089/can.2020.0025. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 137. TÜZÜN B. Investigation of the molecules obtained from marijuana: Computational study of spectral, structural and docking. J. Phys. Theor. Chem. (IAU Iran) 2020;16:59–74. [ Google Scholar ]
- 138. Baroi S., Saha A., Bachar R., Bachar S.C. Cannabinoid as potential aromatase inhibitor through molecular modeling and screening for anti-cancer activity. Dhaka Univ. J. Pharm. Sci. 2020;19:47–58. doi: 10.3329/dujps.v19i1.47818. [ DOI ] [ Google Scholar ]
- 139. Metibemu D.S. 3D-QSAR and Molecular Docking Approaches for the Identification of Phyto-Inhibitors of Hsp90. LIANBS. 2022;11:3871–3886. [ Google Scholar ]
- 140. Adeniran O.Y., Ayorinde O., Boboye S.O. Virtual high-throughput screening (VHTS), three-dimensional quantitative structure-activity and relationship (3D-QSAR) and molecular docking studies of novel phyto-inhibtors of topoisomerase II alpha. GSC Biol. Pharm. Sci. 2021;15:072–082. doi: 10.30574/gscbps.2021.15.2.0099. [ DOI ] [ Google Scholar ]
- 141. Zaka M., Sehgal S.A., Shafique S., Abbasi B.H. Comparative in silico analyses of Cannabis sativa, Prunella vulgaris and Withania somnifera compounds elucidating the medicinal properties against rheumatoid arthritis. J. Mol. Graph. Model. 2017;74:296–304. doi: 10.1016/j.jmgm.2017.04.013. [ DOI ] [ PubMed ] [ Google Scholar ]
- 142. Bouchentouf S., Ghalem S., Allali H., Missoum N. Investigating the Inhibition of 5-LO Enzyme by Main Cannabinoids Contained in Cannabis safiva and Seized Resin Cannabis by Using Molecular Modeling. Insights Enzym. Res. 2017;1:10. [ Google Scholar ]
- 143. Gao J., Li T., Chen D., Gu H., Mao X. Identification and molecular docking of antioxidant peptides from hemp seed protein hydrolysates. Lwt. 2021;147:111453. doi: 10.1016/j.lwt.2021.111453. [ DOI ] [ Google Scholar ]
- 144. Girgih A.T., He R., Aluko R.E. Kinetics and molecular docking studies of the inhibitions of angiotensin converting enzyme and renin activities by hemp seed (Cannabis sativa L.) peptides. J. Agric. Food Chem. 2014;62:4135–4144. doi: 10.1021/jf5002606. [ DOI ] [ PubMed ] [ Google Scholar ]
- 145. Ngamsuk S., Huang T.-C., Hsu J.-L. ACE inhibitory activity and molecular docking of gac seed protein hydrolysate purified by HILIC and RP-HPLC. Molecules. 2020;25:4635. doi: 10.3390/molecules25204635. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 146. Li Y., Ding Y., Xiao W., Zhu J.-B. Investigation on the active ingredient and mechanism of Cannabis sativa L. for treating epilepsy based on network pharmacology. Biotechnol. Biotechnol. Equip. 2021;35:994–1009. doi: 10.1080/13102818.2021.1942208. [ DOI ] [ Google Scholar ]
- 147. di Giacomo V., Chiavaroli A., Recinella L., Orlando G., Cataldi A., Rapino M., Di Valerio V., Ronci M., Leone S., Brunetti L. Antioxidant and neuroprotective effects induced by cannabidiol and cannabigerol in rat CTX-TNA2 astrocytes and isolated cortexes. Int. J. Mol. Sci. 2020;21:3575. doi: 10.3390/ijms21103575. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 148. Sarkar I., Sen G., Bhattacharya M., Bhattacharyya S., Sen A. In silico inquest reveals the efficacy of Cannabis in the treatment of post-Covid-19 related neurodegeneration. J. Biomol. Struct. Dyn. 2022;40:8030–8039. doi: 10.1080/07391102.2021.1905556. [ DOI ] [ PubMed ] [ Google Scholar ]
- 149. Onoda T., Li W., Sasaki T., Miyake M., Higai K., Koike K. Identification and evaluation of magnolol and chrysophanol as the principle protein tyrosine phosphatase-1B inhibitory compounds in a Kampo medicine, Masiningan. J. Ethnopharmacol. 2016;186:84–90. doi: 10.1016/j.jep.2016.03.063. [ DOI ] [ PubMed ] [ Google Scholar ]
- 150. Kazemi F., Karimi I., Yousofvand N. Molecular docking study of lignanamides from Cannabis sativa against P-glycoprotein. Silico Pharmacol. 2021;9:1–7. doi: 10.1007/s40203-020-00066-7. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 151. Holgado M., Martín-Banderas L., Álvarez-Fuentes J., Fernández-Arévalo M. Neuroprotective effect of cannabinoids nanoplatforms in neurodegenerative diseases. J. Drug Deliv. Sci. Technol. 2017;42:84–93. doi: 10.1016/j.jddst.2017.04.023. [ DOI ] [ Google Scholar ]
- 152. Marsh D.T., Smid S.D. Cannabis Phytochemicals: A Review of Phytocannabinoid Chemistry and Bioactivity as Neuroprotective Agents. Aust. J. Chem. 2020;74:388–404. doi: 10.1071/CH20183. [ DOI ] [ Google Scholar ]
- 153. Dreno B., Araviiskaia E., Berardesca E., Bieber T., Hawk J., Sanchez-Viera M., Wolkenstein P. The science of dermocosmetics and its role in dermatology. J. Eur. Acad. Dermatol. Venereol. 2014;28:1409–1417. doi: 10.1111/jdv.12497. [ DOI ] [ PubMed ] [ Google Scholar ]
- 154. Kim J.K., Heo H.-Y., Park S., Kim H., Oh J.J., Sohn E.-H., Jung S.-H., Lee K. Characterization of Phenethyl Cinnamamide Compounds from Hemp Seed and Determination of Their Melanogenesis Inhibitory Activity. ACS Omega. 2021;6:31945–31954. doi: 10.1021/acsomega.1c04727. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 155. Manosroi A., Chankhampan C., Kietthanakorn B.O., Ruksiriwanich W., Chaikul P., Boonpisuttinant K., Sainakham M., Manosroi W., Tangjai T., Manosroi J. Pharmaceutical and cosmeceutical biological activities of hemp (Cannabis sativa L. var. sativa) leaf and seed extracts. Chiang Mai J. Sci. 2019;46:180–195. [ Google Scholar ]
- 156. Yan X., Tang J., dos Santos Passos C., Nurisso A., Simoes-Pires C.A., Ji M., Lou H., Fan P. Characterization of lignanamides from hemp (Cannabis sativa L.) seed and their antioxidant and acetylcholinesterase inhibitory activities. J. Agric. Food Chem. 2015;63:10611–10619. doi: 10.1021/acs.jafc.5b05282. [ DOI ] [ PubMed ] [ Google Scholar ]
- 157. Teh S.-S., Bekhit A.E.-D.A., Carne A., Birch J. Antioxidant and ACE-inhibitory activities of hemp (Cannabis sativa L.) protein hydrolysates produced by the proteases AFP, HT, Pro-G, actinidin and zingibain. Food Chem. 2016;203:199–206. doi: 10.1016/j.foodchem.2016.02.057. [ DOI ] [ PubMed ] [ Google Scholar ]
- 158. Frassinetti S., Gabriele M., Moccia E., Longo V., Di Gioia D. Antimicrobial and antibiofilm activity of Cannabis sativa L. seeds extract against Staphylococcus aureus and growth effects on probiotic Lactobacillus spp. Lwt. 2020;124:109149. doi: 10.1016/j.lwt.2020.109149. [ DOI ] [ Google Scholar ]
- 159. Anjum M. 35. Evaluation of antimicrobial activity and ethnobotanical study of Cannabis sativa L. Pure Appl. Biol. (PAB) 2018;7:706–713. [ Google Scholar ]
- 160. Coetzee C., Levendal R.-A., Van de Venter M., Frost C. Anticoagulant effects of a Cannabis extract in an obese rat model. Phytomedicine. 2007;14:333–337. doi: 10.1016/j.phymed.2006.02.004. [ DOI ] [ PubMed ] [ Google Scholar ]
- 161. Al Khoury A., Sleiman R., Atoui A., Hindieh P., Maroun R.G., Bailly J.-D., El Khoury A. Antifungal and anti-aflatoxigenic properties of organs of Cannabis sativa L.: Relation to phenolic content and antioxidant capacities. Arch. Microbiol. 2021;203:4485–4492. doi: 10.1007/s00203-021-02444-x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 162. Rossi P., Cappelli A., Marinelli O., Valzano M., Pavoni L., Bonacucina G., Petrelli R., Pompei P., Mazzara E., Ricci I. Mosquitocidal and anti-inflammatory properties of the essential oils obtained from monoecious, male, and female inflorescences of hemp (Cannabis sativa L.) and their encapsulation in nanoemulsions. Molecules. 2020;25:3451. doi: 10.3390/molecules25153451. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 163. Nafis A., Kasrati A., Jamali C.A., Mezrioui N., Setzer W., Abbad A., Hassani L. Antioxidant activity and evidence for synergism of Cannabis sativa (L.) essential oil with antimicrobial standards. Ind. Crops Prod. 2019;137:396–400. doi: 10.1016/j.indcrop.2019.05.032. [ DOI ] [ Google Scholar ]
- 164. Mechqoq H., Hourfane S., Yaagoubi M.E., Hamdaoui A.E., Msanda F., Almeida J.R.G.d.S., Rocha J.M., Aouad N.E. Phytochemical Screening, and In Vitro Evaluation of the Antioxidant and Dermocosmetic Activities of Four Moroccan Plants: Halimium antiatlanticum, Adenocarpus artemisiifolius, Pistacia lentiscus and Leonotis nepetifolia. Cosmetics. 2022;9:94. doi: 10.3390/cosmetics9050094. [ DOI ] [ Google Scholar ]
- 165. Mirzamohammad E., Alirezalu A., Alirezalu K., Norozi A., Ansari A. Improvement of the antioxidant activity, phytochemicals, and cannabinoid compounds of Cannabis sativa by salicylic acid elicitor. Food Sci. Nutr. 2021;9:6873–6881. doi: 10.1002/fsn3.2643. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 166. Saura-Calixto F., Serrano J., Pérez-Jiménez J. Beer in Health and Disease Prevention. Elsevier; Amsterdam, The Netherlands: 2009. What contribution is beer to the intake of antioxidants in the diet; pp. 441–448. [ Google Scholar ]
- 167. Shahidi F., Janitha P., Wanasundara P. Phenolic antioxidants. Crit. Rev. Food Sci. Nutr. 1992;32:67–103. doi: 10.1080/10408399209527581. [ DOI ] [ PubMed ] [ Google Scholar ]
- 168. Smeriglio A., Alloisio S., Raimondo F.M., Denaro M., Xiao J., Cornara L., Trombetta D. Essential oil of Citrus lumia Risso: Phytochemical profile, antioxidant properties and activity on the central nervous system. Food Chem. Toxicol. 2018;119:407–416. doi: 10.1016/j.fct.2017.12.053. [ DOI ] [ PubMed ] [ Google Scholar ]
- 169. Novak J., Zitterl-Eglseer K., Deans S.G., Franz C.M. Essential oils of different cultivars of Cannabis sativa L. and their antimicrobial activity. Flavour Fragr. J. 2001;16:259–262. doi: 10.1002/ffj.993. [ DOI ] [ Google Scholar ]
- 170. El Hamdaoui A., Msanda F., Boubaker H., Leach D., Bombarda I., Vanloot P., El Aouad N., Abbad A., Boudyach E., Achemchem F. Essential oil composition, antioxidant and antibacterial activities of wild and cultivated Lavandula mairei Humbert. Biochem. Syst. Ecol. 2018;76:1–7. doi: 10.1016/j.bse.2017.11.004. [ DOI ] [ Google Scholar ]
- 171. Prabuseenivasan S., Jayakumar M., Ignacimuthu S. In vitro antibacterial activity of some plant essential oils. BMC Complement. Altern. Med. 2006;6:1–8. doi: 10.1186/1472-6882-6-39. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 172. El Yaagoubi M., Mechqoq H., Ortiz S., Cavaleiro C., Lecsö-Bornet M., Pereira C.G., Rodrigues M.J., Custódio L., El Mousadik A., Picot L. Chemical Composition and Biological Screening of the Essential Oils of Micromeria macrosiphon and M. arganietorum (Lamiaceae) Chem. Biodivers. 2021;18:e2100653. doi: 10.1002/cbdv.202100653. [ DOI ] [ PubMed ] [ Google Scholar ]
- 173. Xiong W.-T., Gu L., Wang C., Sun H.-X., Liu X. Anti-hyperglycemic and hypolipidemic effects of Cistanche tubulosa in type 2 diabetic db/db mice. J. Ethnopharmacol. 2013;150:935–945. doi: 10.1016/j.jep.2013.09.027. [ DOI ] [ PubMed ] [ Google Scholar ]
- 174. Agarwal P., Gupta R. Alpha-amylase inhibition can treat diabetes mellitus. Res. Rev. J. Med. Health Sci. 2016;5:1–8. [ Google Scholar ]
- 175. Kajaria D., Tripathi J., Tripathi Y.B., Tiwari S. In-vitro α amylase and glycosidase inhibitory effect of ethanolic extract of antiasthmatic drug—Shirishadi. J. Adv. Pharm. Technol. Res. 2013;4:206. doi: 10.4103/2231-4040.121415. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 176. Ligresti A., Moriello A.S., Starowicz K., Matias I., Pisanti S., De Petrocellis L., Laezza C., Portella G., Bifulco M., Di Marzo V. Antitumor activity of plant cannabinoids with emphasis on the effect of cannabidiol on human breast carcinoma. J. Pharmacol. Exp. Ther. 2006;318:1375–1387. doi: 10.1124/jpet.106.105247. [ DOI ] [ PubMed ] [ Google Scholar ]
- 177. Sarfaraz S., Afaq F., Adhami V.M., Malik A., Mukhtar H. Cannabinoid receptor agonist-induced apoptosis of human prostate cancer cells LNCaP proceeds through sustained activation of ERK1/2 leading to G1 cell cycle arrest. J. Biol. Chem. 2006;281:39480–39491. doi: 10.1074/jbc.M603495200. [ DOI ] [ PubMed ] [ Google Scholar ]
- 178. Lukhele S.T., Motadi L.R. Cannabidiol rather than Cannabis sativa extracts inhibit cell growth and induce apoptosis in cervical cancer cells. BMC Complement. Altern. Med. 2016;16:1–16. doi: 10.1186/s12906-016-1280-0. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 179. Marcu J.P., Christian R.T., Lau D., Zielinski A.J., Horowitz M.P., Lee J., Pakdel A., Allison J., Limbad C., Moore D.H. Cannabidiol Enhances the Inhibitory Effects of Δ9-Tetrahydrocannabinol on Human Glioblastoma Cell Proliferation and SurvivalCannabinoid Synergy Inhibits Glioblastoma Cell Growth. Mol. Cancer Ther. 2010;9:180–189. doi: 10.1158/1535-7163.MCT-09-0407. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 180. Cianchi F., Papucci L., Schiavone N., Lulli M., Magnelli L., Vinci M.C., Messerini L., Manera C., Ronconi E., Romagnani P. Cannabinoid receptor activation induces apoptosis through tumor necrosis factor α–mediated ceramide de novo synthesis in colon cancer cells. Clin. Cancer Res. 2008;14:7691–7700. doi: 10.1158/1078-0432.CCR-08-0799. [ DOI ] [ PubMed ] [ Google Scholar ]
- 181. McKallip R.J., Lombard C., Fisher M., Martin B.R., Ryu S., Grant S., Nagarkatti P.S., Nagarkatti M. Targeting CB2 cannabinoid receptors as a novel therapy to treat malignant lymphoblastic disease. Blood J. Am. Soc. Hematol. 2002;100:627–634. doi: 10.1182/blood-2002-01-0098. [ DOI ] [ PubMed ] [ Google Scholar ]
- 182. Armstrong J.L., Hill D.S., McKee C.S., Hernandez-Tiedra S., Lorente M., Lopez-Valero I., Anagnostou M.E., Babatunde F., Corazzari M., Redfern C.P. Exploiting cannabinoid-induced cytotoxic autophagy to drive melanoma cell death. J. Investig. Dermatol. 2015;135:1629–1637. doi: 10.1038/jid.2015.45. [ DOI ] [ PubMed ] [ Google Scholar ]
- 183. Borrelli F., Pagano E., Romano B., Panzera S., Maiello F., Coppola D., De Petrocellis L., Buono L., Orlando P., Izzo A.A. Colon carcinogenesis is inhibited by the TRPM8 antagonist cannabigerol, a Cannabis-derived non-psychotropic cannabinoid. Carcinogenesis. 2014;35:2787–2797. doi: 10.1093/carcin/bgu205. [ DOI ] [ PubMed ] [ Google Scholar ]
- 184. Galanti G., Fisher T., Kventsel I., Shoham J., Gallily R., Mechoulam R., Lavie G., Amariglio N., Rechavi G., Toren A. Δ9-Tetrahydrocannabinol inhibits cell cycle progression by downregulation of E2F1 in human glioblastoma multiforme cells. Acta Oncol. 2008;47:1062–1070. doi: 10.1080/02841860701678787. [ DOI ] [ PubMed ] [ Google Scholar ]
- 185. Galve-Roperh I., Sánchez C., Cortés M.L., del Pulgar T.G., Izquierdo M., Guzmán M. Anti-tumoral action of cannabinoids: Involvement of sustained ceramide accumulation and extracellular signal-regulated kinase activation. Nat. Med. 2000;6:313–319. doi: 10.1038/73171. [ DOI ] [ PubMed ] [ Google Scholar ]
- 186. McAllister S.D., Murase R., Christian R.T., Lau D., Zielinski A.J., Allison J., Almanza C., Pakdel A., Lee J., Limbad C. Pathways mediating the effects of cannabidiol on the reduction of breast cancer cell proliferation, invasion, and metastasis. Breast Cancer Res. Treat. 2011;129:37–47. doi: 10.1007/s10549-010-1177-4. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 187. Solinas M., Massi P., Cinquina V., Valenti M., Bolognini D., Gariboldi M., Monti E., Rubino T., Parolaro D. Cannabidiol, a non-psychoactive cannabinoid compound, inhibits proliferation and invasion in U87-MG and T98G glioma cells through a multitarget effect. PLoS ONE. 2013;8:e76918. doi: 10.1371/journal.pone.0076918. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 188. Klein T.W. Cannabinoid-based drugs as anti-inflammatory therapeutics. Nat. Rev. Immunol. 2005;5:400–411. doi: 10.1038/nri1602. [ DOI ] [ PubMed ] [ Google Scholar ]
- 189. Nagarkatti P., Pandey R., Rieder S.A., Hegde V.L., Nagarkatti M. Cannabinoids as novel anti-inflammatory drugs. Future Med. Chem. 2009;1:1333–1349. doi: 10.4155/fmc.09.93. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 190. Wood C., Duparc N., Leblanc V., Cunin-Roy C. L’hypnose et la douleur. Médecine Clin. Pour Les Pédiatres. 2004;11:40–44. [ Google Scholar ]
- 191. Munch G. Le Cannabis, Les Deux Versants: Drogue et Médicament. Université de Lorraine; Lorraine, France: 2015. [ Google Scholar ]
- 192. Jeannin C. Master Thesis. University of Liege; Liege, Belgium: 2020. Évaluation et Prise en Charge de la Douleur Chez le Lapin de Compagnie: Comment les Optimiser en L’état Actuel des Connaissances? [ Google Scholar ]
- 193. Li J., Wang G., Qin Y., Zhang X., Wang H.-F., Liu H.-W., Zhu L.-J., Yao X.-S. Neuroprotective constituents from the aerial parts of Cannabis sativa L. subsp. sativa. RSC Adv. 2020;10:32043–32049. doi: 10.1039/D0RA04565A. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 194. Landucci E., Mazzantini C., Lana D., Davolio P.L., Giovannini M.G., Pellegrini-Giampietro D.E. Neuroprotective effects of cannabidiol but not Δ9-Tetrahydrocannabinol in rat hippocampal slices exposed to oxygen-glucose deprivation: Studies with Cannabis extracts and selected cannabinoids. Int. J. Mol. Sci. 2021;22:9773. doi: 10.3390/ijms22189773. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 195. Esposito G., Scuderi C., Savani C., Steardo Jr L., De Filippis D., Cottone P., Iuvone T., Cuomo V., Steardo L. Cannabidiol in vivo blunts β-amyloid induced neuroinflammation by suppressing IL-1β and iNOS expression. Br. J. Pharmacol. 2007;151:1272–1279. doi: 10.1038/sj.bjp.0707337. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 196. Perez M., Benitez S.U., Cartarozzi L.P., Del Bel E., Guimaraes F.S., Oliveira A.L. Neuroprotection and reduction of glial reaction by cannabidiol treatment after sciatic nerve transection in neonatal rats. Eur. J. Neurosci. 2013;38:3424–3434. doi: 10.1111/ejn.12341. [ DOI ] [ PubMed ] [ Google Scholar ]
- 197. Friedman L.K., Wongvravit J.P. Anticonvulsant and neuroprotective effects of cannabidiol during the juvenile period. J. Neuropathol. Exp. Neurol. 2018;77:904–919. doi: 10.1093/jnen/nly069. [ DOI ] [ PubMed ] [ Google Scholar ]
- 198. Devinsky O., Marsh E., Friedman D., Thiele E., Laux L., Sullivan J., Miller I., Flamini R., Wilfong A., Filloux F. Cannabidiol in patients with treatment-resistant epilepsy: An open-label interventional trial. Lancet Neurol. 2016;15:270–278. doi: 10.1016/S1474-4422(15)00379-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 199. Farrelly A.M., Vlachou S., Grintzalis K. Efficacy of phytocannabinoids in epilepsy treatment: Novel approaches and recent advances. Int. J. Environ. Res. Public Health. 2021;18:3993. doi: 10.3390/ijerph18083993. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 200. Stone N.L., Murphy A.J., England T.J., O’Sullivan S.E. A systematic review of minor phytocannabinoids with promising neuroprotective potential. Br. J. Pharmacol. 2020;177:4330–4352. doi: 10.1111/bph.15185. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 201. Dunn L.B., Damesyn M., Moore A.A., Reuben D.B., Greendale G.A. Does estrogen prevent skin aging?: Results from the first National Health and Nutrition Examination Survey (NHANES I) Arch. Dermatol. 1997;133:339–342. doi: 10.1001/archderm.1997.03890390077010. [ DOI ] [ PubMed ] [ Google Scholar ]
- 202. Gaisey J., Narouze S.N. Cannabinoids and Pain. Springer; Berlin/Heidelberg, Germany: 2021. Dronabinol (Marinol®) pp. 105–107. [ Google Scholar ]
- 203. Abuhasira R., Shbiro L., Landschaft Y. Medical use of cannabis and cannabinoids containing products–Regulations in Europe and North America. Eur. J. Intern. Med. 2018;49:2–6. doi: 10.1016/j.ejim.2018.01.001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 204. Babson K.A., Sottile J., Morabito D. Cannabis, cannabinoids, and sleep: A review of the literature. Curr. Psychiatry Rep. 2017;19:1–12. doi: 10.1007/s11920-017-0775-9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 205. Sekar K., Pack A. Epidiolex as adjunct therapy for treatment of refractory epilepsy: A comprehensive review with a focus on adverse effects. F1000Research. 2019;8:8. doi: 10.12688/f1000research.16515.1. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
- View on publisher site
- PDF (3.2 MB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Journal of Cannabis Research
Aims and scope.
The Journal of Cannabis Research is an international, fully open access, peer-reviewed journal covering all topics pertaining to cannabis , including original research, perspectives, commentaries and protocols. Our goal is to provide an accessible outlet for expert interdisciplinary discourse on cannabis research.

We are recruiting!
Journal of Cannabis Research is recruiting Associate Editors. As the growth of the journal continues, we are looking to expand our editorial team. If you have experience in any form of cannabis research, we would like to hear from you. Please follow the link below to find out more about the role and apply.
Collection Call for Papers: New developments in Cannabis sativa science: agronomy, plant biology, and molecular insights
Register for the Webinar Series hosted by the Institute of Cannabis Research and watch a comprehensive archive of cutting edge science presented by experts across the spectrum of cannabis research. The Cannabis Research Series hosted in collaboration with the Lambert Center at Thomas Jefferson University Cannabis Plant Science and Cultivation , hosted in collaboration with the Volcani Institute in Israel.
The Two Sides of Hemp
About the Institute
The Journal of Cannabis Research is the official publication of the Institute of Cannabis Research (ICR), which was established in June 2016 through an innovative partnership between Colorado State University Pueblo, the state of Colorado, and Pueblo County.
The ICR is the first US multi-disciplinary cannabis research center at a regional, comprehensive institution. The primary goal of the ICR is to conduct or fund research related to cannabis and publicly disseminate the results of the research, which it does so in partnership with the Journal. It also advises other Colorado-based higher education institutions on the development of a cannabis-related curriculum and supports the translation of discoveries into innovative applications that improve lives.
Find out more about the the Institute via the link below, as well as the Colorado state University–Pueblo website and Institute's research funding outcomes .

Affiliated with

An official publication of the Institute of Cannabis Research
- Editorial Board
- Instructions for Authors
- Instructions for Editors
- Sign up for article alerts and news from this journal
Annual Journal Metrics
Citation Impact 2023 Journal Impact Factor: 4.1 5-year Journal Impact Factor: 3.8 Source Normalized Impact per Paper (SNIP): 0.894 SCImago Journal Rank (SJR): 0.692
Speed 2023 Submission to first editorial decision (median days): 22 Submission to acceptance (median days): 211
Usage 2023 Downloads: 596,678 Altmetric mentions: 1,834
- More about our metrics
ISSN: 2522-5782
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Research article
- Open access
- Published: 04 February 2020
Marijuana legalization and historical trends in marijuana use among US residents aged 12–25: results from the 1979–2016 National Survey on drug use and health
- Xinguang Chen 1 ,
- Xiangfan Chen 2 &
- Hong Yan 2
BMC Public Health volume 20 , Article number: 156 ( 2020 ) Cite this article
112k Accesses
83 Citations
81 Altmetric
Metrics details
Marijuana is the most commonly used illicit drug in the United States. More and more states legalized medical and recreational marijuana use. Adolescents and emerging adults are at high risk for marijuana use. This ecological study aims to examine historical trends in marijuana use among youth along with marijuana legalization.
Data ( n = 749,152) were from the 31-wave National Survey on Drug Use and Health (NSDUH), 1979–2016. Current marijuana use, if use marijuana in the past 30 days, was used as outcome variable. Age was measured as the chronological age self-reported by the participants, period was the year when the survey was conducted, and cohort was estimated as period subtracted age. Rate of current marijuana use was decomposed into independent age, period and cohort effects using the hierarchical age-period-cohort (HAPC) model.
After controlling for age, cohort and other covariates, the estimated period effect indicated declines in marijuana use in 1979–1992 and 2001–2006, and increases in 1992–2001 and 2006–2016. The period effect was positively and significantly associated with the proportion of people covered by Medical Marijuana Laws (MML) (correlation coefficients: 0.89 for total sample, 0.81 for males and 0.93 for females, all three p values < 0.01), but was not significantly associated with the Recreational Marijuana Laws (RML). The estimated cohort effect showed a historical decline in marijuana use in those who were born in 1954–1972, a sudden increase in 1972–1984, followed by a decline in 1984–2003.
The model derived trends in marijuana use were coincident with the laws and regulations on marijuana and other drugs in the United States since the 1950s. With more states legalizing marijuana use in the United States, emphasizing responsible use would be essential to protect youth from using marijuana.
Peer Review reports
Introduction
Marijuana use and laws in the united states.
Marijuana is one of the most commonly used drugs in the United States (US) [ 1 ]. In 2015, 8.3% of the US population aged 12 years and older used marijuana in the past month; 16.4% of adolescents aged 12–17 years used in lifetime and 7.0% used in the past month [ 2 ]. The effects of marijuana on a person’s health are mixed. Despite potential benefits (e.g., relieve pain) [ 3 ], using marijuana is associated with a number of adverse effects, particularly among adolescents. Typical adverse effects include impaired short-term memory, cognitive impairment, diminished life satisfaction, and increased risk of using other substances [ 4 ].
Since 1937 when the Marijuana Tax Act was issued, a series of federal laws have been subsequently enacted to regulate marijuana use, including the Boggs Act (1952), Narcotics Control Act (1956), Controlled Substance Act (1970), and Anti-Drug Abuse Act (1986) [ 5 , 6 ]. These laws regulated the sale, possession, use, and cultivation of marijuana [ 6 ]. For example, the Boggs Act increased the punishment of marijuana possession, and the Controlled Substance Act categorized the marijuana into the Schedule I Drugs which have a high potential for abuse, no medical use, and not safe to use without medical supervision [ 5 , 6 ]. These federal laws may have contributed to changes in the historical trend of marijuana use among youth.
Movements to decriminalize and legalize marijuana use
Starting in the late 1960s, marijuana decriminalization became a movement, advocating reformation of federal laws regulating marijuana [ 7 ]. As a result, 11 US states had taken measures to decriminalize marijuana use by reducing the penalty of possession of small amount of marijuana [ 7 ].
The legalization of marijuana started in 1993 when Surgeon General Elder proposed to study marijuana legalization [ 8 ]. California was the first state that passed Medical Marijuana Laws (MML) in 1996 [ 9 ]. After California, more and more states established laws permitting marijuana use for medical and/or recreational purposes. To date, 33 states and the District of Columbia have established MML, including 11 states with recreational marijuana laws (RML) [ 9 ]. Compared with the legalization of marijuana use in the European countries which were more divided that many of them have medical marijuana registered as a treatment option with few having legalized recreational use [ 10 , 11 , 12 , 13 ], the legalization of marijuana in the US were more mixed with 11 states legalized medical and recreational use consecutively, such as California, Nevada, Washington, etc. These state laws may alter people’s attitudes and behaviors, finally may lead to the increased risk of marijuana use, particularly among young people [ 13 ]. Reported studies indicate that state marijuana laws were associated with increases in acceptance of and accessibility to marijuana, declines in perceived harm, and formation of new norms supporting marijuana use [ 14 ].
Marijuana harm to adolescents and young adults
Adolescents and young adults constitute a large proportion of the US population. Data from the US Census Bureau indicate that approximately 60 million of the US population are in the 12–25 years age range [ 15 ]. These people are vulnerable to drugs, including marijuana [ 16 ]. Marijuana is more prevalent among people in this age range than in other ages [ 17 ]. One well-known factor for explaining the marijuana use among people in this age range is the theory of imbalanced cognitive and physical development [ 4 ]. The delayed brain development of youth reduces their capability to cognitively process social, emotional and incentive events against risk behaviors, such as marijuana use [ 18 ]. Understanding the impact of marijuana laws on marijuana use among this population with a historical perspective is of great legal, social and public health significance.
Inconsistent results regarding the impact of marijuana laws on marijuana use
A number of studies have examined the impact of marijuana laws on marijuana use across the world, but reported inconsistent results [ 13 ]. Some studies reported no association between marijuana laws and marijuana use [ 14 , 19 , 20 , 21 , 22 , 23 , 24 , 25 ], some reported a protective effect of the laws against marijuana use [ 24 , 26 ], some reported mixed effects [ 27 , 28 ], while some others reported a risk effect that marijuana laws increased marijuana use [ 29 , 30 ]. Despite much information, our review of these reported studies revealed several limitations. First of all, these studies often targeted a short time span, ignoring the long period trend before marijuana legalization. Despite the fact that marijuana laws enact in a specific year, the process of legalization often lasts for several years. Individuals may have already changed their attitudes and behaviors before the year when the law is enacted. Therefore, it may not be valid when comparing marijuana use before and after the year at a single time point when the law is enacted and ignoring the secular historical trend [ 19 , 30 , 31 ]. Second, many studies adapted the difference-in-difference analytical approach designated for analyzing randomized controlled trials. No US state is randomized to legalize the marijuana laws, and no state can be established as controls. Thus, the impact of laws cannot be efficiently detected using this approach. Third, since marijuana legalization is a public process, and the information of marijuana legalization in one state can be easily spread to states without the marijuana laws. The information diffusion cannot be ruled out, reducing the validity of the non-marijuana law states as the controls to compare the between-state differences [ 31 ].
Alternatively, evidence derived based on a historical perspective may provide new information regarding the impact of laws and regulations on marijuana use, including state marijuana laws in the past two decades. Marijuana users may stop using to comply with the laws/regulations, while non-marijuana users may start to use if marijuana is legal. Data from several studies with national data since 1996 demonstrate that attitudes, beliefs, perceptions, and use of marijuana among people in the US were associated with state marijuana laws [ 29 , 32 ].
Age-period-cohort modeling: looking into the past with recent data
To investigate historical trends over a long period, including the time period with no data, we can use the classic age-period-cohort modeling (APC) approach. The APC model can successfully discompose the rate or prevalence of marijuana use into independent age, period and cohort effects [ 33 , 34 ]. Age effect refers to the risk associated with the aging process, including the biological and social accumulation process. Period effect is risk associated with the external environmental events in specific years that exert effect on all age groups, representing the unbiased historical trend of marijuana use which controlling for the influences from age and birth cohort. Cohort effect refers to the risk associated with the specific year of birth. A typical example is that people born in 2011 in Fukushima, Japan may have greater risk of cancer due to the nuclear disaster [ 35 ], so a person aged 80 in 2091 contains the information of cancer risk in 2011 when he/she was born. Similarly, a participant aged 25 in 1979 contains information on the risk of marijuana use 25 years ago in 1954 when that person was born. With this method, we can describe historical trends of marijuana use using information stored by participants in older ages [ 33 ]. The estimated period and cohort effects can be used to present the unbiased historical trend of specific topics, including marijuana use [ 34 , 36 , 37 , 38 ]. Furthermore, the newly established hierarchical APC (HAPC) modeling is capable of analyzing individual-level data to provide more precise measures of historical trends [ 33 ]. The HAPC model has been used in various fields, including social and behavioral science, and public health [ 39 , 40 ].
Several studies have investigated marijuana use with APC modeling method [ 17 , 41 , 42 ]. However, these studies covered only a small portion of the decades with state marijuana legalization [ 17 , 42 ]. For example, the study conducted by Miech and colleagues only covered periods from 1985 to 2009 [ 17 ]. Among these studies, one focused on a longer state marijuana legalization period, but did not provide detailed information regarding the impact of marijuana laws because the survey was every 5 years and researchers used a large 5-year age group which leads to a wide 10-year birth cohort. The averaging of the cohort effects in 10 years could reduce the capability of detecting sensitive changes of marijuana use corresponding to the historical events [ 41 ].
Purpose of the study
In this study, we examined the historical trends in marijuana use among youth using HAPC modeling to obtain the period and cohort effects. These two effects provide unbiased and independent information to characterize historical trends in marijuana use after controlling for age and other covariates. We conceptually linked the model-derived time trends to both federal and state laws/regulations regarding marijuana and other drug use in 1954–2016. The ultimate goal is to provide evidence informing federal and state legislation and public health decision-making to promote responsible marijuana use and to protect young people from marijuana use-related adverse consequences.
Materials and methods
Data sources and study population.
Data were derived from 31 waves of National Survey on Drug Use and Health (NSDUH), 1979–2016. NSDUH is a multi-year cross-sectional survey program sponsored by the Substance Abuse and Mental Health Services Administration. The survey was conducted every 3 years before 1990, and annually thereafter. The aim is to provide data on the use of tobacco, alcohol, illicit drug and mental health among the US population.
Survey participants were noninstitutionalized US civilians 12 years of age and older. Participants were recruited by NSDUH using a multi-stage clustered random sampling method. Several changes were made to the NSDUH after its establishment [ 43 ]. First, the name of the survey was changed from the National Household Survey on Drug Abuse (NHSDA) to NSDUH in 2002. Second, starting in 2002, adolescent participants receive $30 as incentives to improve the response rate. Third, survey mode was changed from personal interviews with self-enumerated answer sheets (before 1999) to the computer-assisted person interviews (CAPI) and audio computer-assisted self-interviews (ACASI) (since 1999). These changes may confound the historical trends [ 43 ], thus we used two dummy variables as covariates, one for the survey mode change in 1999 and another for the survey method change in 2002 to control for potential confounding effect.
Data acquisition
Data were downloaded from the designated website ( https://nsduhweb.rti.org/respweb/homepage.cfm ). A database was used to store and merge the data by year for analysis. Among all participants, data for those aged 12–25 years ( n = 749,152) were included. We excluded participants aged 26 and older because the public data did not provide information on single or two-year age that was needed for HAPC modeling (details see statistical analysis section). We obtained approval from the Institutional Review Board at the University of Florida to conduct this study.
Variables and measurements
Current marijuana use: the dependent variable. Participants were defined as current marijuana users if they reported marijuana use within the past 30 days. We used the variable harmonization method to create a comparable measure across 31-wave NSDUH data [ 44 ]. Slightly different questions were used in NSDUH. In 1979–1993, participants were asked: “When was the most recent time that you used marijuana or hash?” Starting in 1994, the question was changed to “How long has it been since you last used marijuana or hashish?” To harmonize the marijuana use variable, participants were coded as current marijuana users if their response to the question indicated the last time to use marijuana was within past 30 days.
Chronological age, time period and birth cohort were the predictors. (1) Chronological age in years was measured with participants’ age at the survey. APC modeling requires the same age measure for all participants [ 33 ]. Since no data by single-year age were available for participants older than 21, we grouped all participants into two-year age groups. A total of 7 age groups, 12–13, ..., 24–25 were used. (2) Time period was measured with the year when the survey was conducted, including 1979, 1982, 1985, 1988, 1990, 1991... 2016. (3). Birth cohort was the year of birth, and it was measured by subtracting age from the survey year.
The proportion of people covered by MML: This variable was created by dividing the population in all states with MML over the total US population. The proportion was computed by year from 1996 when California first passed the MML to 2016 when a total of 29 states legalized medical marijuana use. The estimated proportion ranged from 12% in 1996 to 61% in 2016. The proportion of people covered by RML: This variable was derived by dividing the population in all states with RML with the total US population. The estimated proportion ranged from 4% in 2012 to 21% in 2016. These two variables were used to quantitatively assess the relationships between marijuana laws and changes in the risk of marijuana use.
Covariates: Demographic variables gender (male/female) and race/ethnicity (White, Black, Hispanic and others) were used to describe the study sample.
Statistical analysis
We estimated the prevalence of current marijuana use by year using the survey estimation method, considering the complex multi-stage cluster random sampling design and unequal probability. A prevalence rate is not a simple indicator, but consisting of the impact of chronological age, time period and birth cohort, named as age, period and cohort effects, respectively. Thus, it is biased if a prevalence rate is directly used to depict the historical trend. HAPC modeling is an epidemiological method capable of decomposing prevalence rate into mutually independent age, period and cohort effects with individual-level data, while the estimated period and cohort effects provide an unbiased measure of historical trend controlling for the effects of age and other covariates. In this study, we analyzed the data using the two-level HAPC cross-classified random-effects model (CCREM) [ 36 ]:
Where M ijk represents the rate of marijuana use for participants in age group i (12–13, 14,15...), period j (1979, 1982,...) and birth cohort k (1954–55, 1956–57...); parameter α i (age effect) was modeled as the fixed effect; and parameters β j (period effect) and γ k (cohort effect) were modeled as random effects; and β m was used to control m covariates, including the two dummy variables assessing changes made to the NSDUH in 1999 and 2002, respectively.
The HAPC modeling analysis was executed using the PROC GLIMMIX. Sample weights were included to obtain results representing the total US population aged 12–25. A ridge-stabilized Newton-Raphson algorithm was used for parameter estimation. Modeling analysis was conducted for the overall sample, stratified by gender. The estimated age effect α i , period β j and cohort γ k (i.e., the log-linear regression coefficients) were directly plotted to visualize the pattern of change.
To gain insight into the relationship between legal events and regulations at the national level, we listed these events/regulations along with the estimated time trends in the risk of marijuana from HAPC modeling. To provide a quantitative measure, we associated the estimated period effect with the proportions of US population living with MML and RML using Pearson correlation. All statistical analyses for this study were conducted using the software SAS, version 9.4 (SAS Institute Inc., Cary, NC).
Sample characteristics
Data for a total of 749,152 participants (12–25 years old) from all 31-wave NSDUH covering a 38-year period were analyzed. Among the total sample (Table 1 ), 48.96% were male and 58.78% were White, 14.84% Black, and 18.40% Hispanic.
Prevalence rate of current marijuana use
As shown in Fig. 1 , the estimated prevalence rates of current marijuana use from 1979 to 2016 show a “V” shaped pattern. The rate was 27.57% in 1979, it declined to 8.02% in 1992, followed by a gradual increase to 14.70% by 2016. The pattern was the same for both male and female with males more likely to use than females during the whole period.
Prevalence rate (%) of current marijuana use among US residents 12 to 25 years of age during 1979–2016, overall and stratified by gender. Derived from data from the 1979–2016 National Survey on Drug Use and Health (NSDUH)
HAPC modeling and results
Estimated age effects α i from the CCREM [ 1 ] for current marijuana use are presented in Fig. 2 . The risk by age shows a 2-phase pattern –a rapid increase phase from ages 12 to 19, followed by a gradually declining phase. The pattern was persistent for the overall sample and for both male and female subsamples.
Age effect for the risk of current marijuana use, overall and stratified by male and female, estimated with hierarchical age-period-cohort modeling method with 31 waves of NSDUH data during 1979–2016. Age effect α i were log-linear regression coefficients estimated using CCREM (1), see text for more details
The estimated period effects β j from the CCREM [ 1 ] are presented in Fig. 3 . The period effect reflects the risk of current marijuana use due to significant events occurring over the period, particularly federal and state laws and regulations. After controlling for the impacts of age, cohort and other covariates, the estimated period effect indicates that the risk of current marijuana use had two declining trends (1979–1992 and 2001–2006), and two increasing trends (1992–2001 and 2006–2016). Epidemiologically, the time trends characterized by the estimated period effects in Fig. 3 are more valid than the prevalence rates presented in Fig. 1 because the former was adjusted for confounders while the later was not.
Period effect for the risk of marijuana use for US adolescents and young adults, overall and by male/female estimated with hierarchical age-period-cohort modeling method and its correlation with the proportion of US population covered by Medical Marijuana Laws and Recreational Marijuana Laws. Period effect β j were log-linear regression coefficients estimated using CCREM (1), see text for more details
Correlation of the period effect with proportions of the population covered by marijuana laws: The Pearson correlation coefficient of the period effect with the proportions of US population covered by MML during 1996–2016 was 0.89 for the total sample, 0.81 for male and 0.93 for female, respectively ( p < 0.01 for all). The correlation between period effect and proportion of US population covered by RML was 0.64 for the total sample, 0.59 for male and 0.49 for female ( p > 0.05 for all).
Likewise, the estimated cohort effects γ k from the CCREM [ 1 ] are presented in Fig. 4 . The cohort effect reflects changes in the risk of current marijuana use over the period indicated by the year of birth of the survey participants after the impacts of age, period and other covariates are adjusted. Results in the figure show three distinctive cohorts with different risk patterns of current marijuana use during 1954–2003: (1) the Historical Declining Cohort (HDC): those born in 1954–1972, and characterized by a gradual and linear declining trend with some fluctuations; (2) the Sudden Increase Cohort (SIC): those born from 1972 to 1984, characterized with a rapid almost linear increasing trend; and (3) the Contemporary Declining Cohort (CDC): those born during 1984 and 2003, and characterized with a progressive declining over time. The detailed results of HAPC modeling analysis were also shown in Additional file 1 : Table S1.

Cohort effect for the risk of marijuana use among US adolescents and young adults born during 1954–2003, overall and by male/female, estimated with hierarchical age-period-cohort modeling method. Cohort effect γ k were log-linear regression coefficients estimated using CCREM (1), see text for more details
This study provides new data regarding the risk of marijuana use in youth in the US during 1954–2016. This is a period in the US history with substantial increases and declines in drug use, including marijuana; accompanied with many ups and downs in legal actions against drug use since the 1970s and progressive marijuana legalization at the state level from the later 1990s till today (see Additional file 1 : Table S2). Findings of the study indicate four-phase period effect and three-phase cohort effect, corresponding to various historical events of marijuana laws, regulations and social movements.
Coincident relationship between the period effect and legal drug control
The period effect derived from the HAPC model provides a net effect of the impact of time on marijuana use after the impact of age and birth cohort were adjusted. Findings in this study indicate that there was a progressive decline in the period effect during 1979 and 1992. This trend was corresponding to a period with the strongest legal actions at the national level, the War on Drugs by President Nixon (1969–1974) President Reagan (1981–1989) [ 45 ], and President Bush (1989) [ 45 ],and the Anti-Drug Abuse Act (1986) [ 5 ].
The estimated period effect shows an increasing trend in 1992–2001. During this period, President Clinton advocated for the use of treatment to replace incarceration (1992) [ 45 ], Surgeon General Elders proposed to study marijuana legalization (1993–1994) [ 8 ], President Clinton’s position of the need to re-examine the entire policy against people who use drugs, and decriminalization of marijuana (2000) [ 45 ] and the passage of MML in eight US states.
The estimated period effect shows a declining trend in 2001–2006. Important laws/regulations include the Student Drug Testing Program promoted by President Bush, and the broadened the public schools’ authority to test illegal drugs among students given by the US Supreme Court (2002) [ 46 ].
The estimated period effect increases in 2006–2016. This is the period when the proportion of the population covered by MML progressively increased. This relation was further proved by a positive correlation between the estimated period effect and the proportion of the population covered by MML. In addition, several other events occurred. For example, over 500 economists wrote an open letter to President Bush, Congress and Governors of the US and called for marijuana legalization (2005) [ 47 ], and President Obama ended the federal interference with the state MML, treated marijuana as public health issues, and avoided using the term of “War on Drugs” [ 45 ]. The study also indicates that the proportion of population covered by RML was positively associated with the period effect although not significant which may be due to the limited number of data points of RML. Future studies may follow up to investigate the relationship between RML and rate of marijuana use.
Coincident relationship between the cohort effect and legal drug control
Cohort effect is the risk of marijuana use associated with the specific year of birth. People born in different years are exposed to different laws, regulations in the past, therefore, the risk of marijuana use for people may differ when they enter adolescence and adulthood. Findings in this study indicate three distinctive cohorts: HDC (1954–1972), SIC (1972–1984) and CDC (1984–2003). During HDC, the overall level of marijuana use was declining. Various laws/regulations of drug use in general and marijuana in particular may explain the declining trend. First, multiple laws passed to regulate the marijuana and other substance use before and during this period remained in effect, for example, the Marijuana Tax Act (1937), the Boggs Act (1952), the Narcotics Control Act (1956) and the Controlled Substance Act (1970). Secondly, the formation of government departments focusing on drug use prevention and control may contribute to the cohort effect, such as the Bureau of Narcotics and Dangerous Drugs (1968) [ 48 ]. People born during this period may be exposed to the macro environment with laws and regulations against marijuana, thus, they may be less likely to use marijuana.
Compared to people born before 1972, the cohort effect for participants born during 1972 and 1984 was in coincidence with the increased risk of using marijuana shown as SIC. This trend was accompanied by the state and federal movements for marijuana use, which may alter the social environment and public attitudes and beliefs from prohibitive to acceptive. For example, seven states passed laws to decriminalize the marijuana use and reduced the penalty for personal possession of small amount of marijuana in 1976 [ 7 ]. Four more states joined the movement in two subsequent years [ 7 ]. People born during this period may have experienced tolerated environment of marijuana, and they may become more acceptable of marijuana use, increasing their likelihood of using marijuana.
A declining cohort CDC appeared immediately after 1984 and extended to 2003. This declining cohort effect was corresponding to a number of laws, regulations and movements prohibiting drug use. Typical examples included the War on Drugs initiated by President Nixon (1980s), the expansion of the drug war by President Reagan (1980s), the highly-publicized anti-drug campaign “Just Say No” by First Lady Nancy Reagan (early 1980s) [ 45 ], and the Zero Tolerance Policies in mid-to-late 1980s [ 45 ], the Anti-Drug Abuse Act (1986) [ 5 ], the nationally televised speech of War on Drugs declared by President Bush in 1989 and the escalated War on Drugs by President Clinton (1993–2001) [ 45 ]. Meanwhile many activities of the federal government and social groups may also influence the social environment of using marijuana. For example, the Federal government opposed to legalize the cultivation of industrial hemp, and Federal agents shut down marijuana sales club in San Francisco in 1998 [ 48 ]. Individuals born in these years grew up in an environment against marijuana use which may decrease their likelihood of using marijuana when they enter adolescence and young adulthood.
This study applied the age-period-cohort model to investigate the independent age, period and cohort effects, and indicated that the model derived trends in marijuana use among adolescents and young adults were coincident with the laws and regulations on marijuana use in the United States since the 1950s. With more states legalizing marijuana use in the United States, emphasizing responsible use would be essential to protect youth from using marijuana.
Limitations
This study has limitations. First, study data were collected through a household survey, which is subject to underreporting. Second, no causal relationship can be warranted using cross-sectional data, and further studies are needed to verify the association between the specific laws/regulation and the risk of marijuana use. Third, data were available to measure single-year age up to age 21 and two-year age group up to 25, preventing researchers from examining the risk of marijuana use for participants in other ages. Lastly, data derived from NSDUH were nation-wide, and future studies are needed to analyze state-level data and investigate the between-state differences. Although a systematic review of all laws and regulations related to marijuana and other drugs is beyond the scope of this study, findings from our study provide new data from a historical perspective much needed for the current trend in marijuana legalization across the nation to get the benefit from marijuana while to protect vulnerable children and youth in the US. It provides an opportunity for stack-holders to make public decisions by reviewing the findings of this analysis together with the laws and regulations at the federal and state levels over a long period since the 1950s.
Availability of data and materials
The data of the study are available from the designated repository ( https://nsduhweb.rti.org/respweb/homepage.cfm ).
Abbreviations
Audio computer-assisted self-interviews
Age-period-cohort modeling
Computer-assisted person interviews
Cross-classified random-effects model
Contemporary Declining Cohort
Hierarchical age-period-cohort
Historical Declining Cohort
Medical Marijuana Laws
National Household Survey on Drug Abuse
National Survey on Drug Use and Health
Recreational Marijuana Laws
Sudden Increase Cohort
The United States
CDC. Marijuana and Public Health. 2017. Available from: https://www.cdc.gov/marijuana/index.htm . Accessed 13 June 2018.
SAMHSA. Results from the 2015 National Survey on Drug Use and Health: Detailed Tables. 2016 [cited 2018 Jan 31]. Available from: https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.htm
Committee on the Health Effects of Marijuana: An Evidence Review and Research Agenda, Board on Population Health and Public Health Practice, Health and Medicine Division, National Academies of Sciences, Engineering, and Medicine. The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. Washington, D.C.: National Academies Press; 2017.
Collins C. Adverse health effects of marijuana use. N Engl J Med. 2014;371(9):879.
PubMed Google Scholar
Belenko SR. Drugs and drug policy in America: a documentary history. Westport: Greenwood Press; 2000.
Google Scholar
Gerber RJ. Legalizing marijuana: Drug policy reform and prohibition politics. Westport: Praeger; 2004.
Single EW. The impact of marijuana decriminalization: an update. J Public Health Policy. 1989:456–66.
Article CAS Google Scholar
SFChronicle. Ex-surgeon general backed legalizing marijuana before it was cool [Internet]. 2016 [cited 2018 Oct 7]. Available from: https://www.sfchronicle.com/business/article/Ex-surgeon-general-backed-legalizing-marijuana-6799348.php
PROCON. 31 Legal Medical Marijuana States and DC. 2018 [cited 2018 Oct 4]. Available from: https://medicalmarijuana.procon.org/view.resource.php?resourceID=000881
Bifulco M, Pisanti S. Medicinal use of cannabis in Europe: the fact that more countries legalize the medicinal use of cannabis should not become an argument for unfettered and uncontrolled use. EMBO Rep. 2015;16(2):130–2.
European Monitoring Centre for Drugs and Drug Addiction. Models for the legal supply of cannabis: recent developments (Perspectives on drugs). 2016. Available from: http://www.emcdda.europa.eu/publications/pods/legal-supply-of-cannabis . Accessed 10 Jan 2020.
European Monitoring Centre for Drugs and Drug Addiction. Cannabis policy: status and recent developments. 2017. Available from: http://www.emcdda.europa.eu/topics/cannabis-policy_en#section2 . Accessed 10 Jan 2020.
Hughes B, Matias J, Griffiths P. Inconsistencies in the assumptions linking punitive sanctions and use of cannabis and new psychoactive substances in Europe. Addiction. 2018;113(12):2155–7.
Article Google Scholar
Anderson DM, Hansen B, Rees DI. Medical marijuana laws and teen marijuana use. Am Law Econ Rev. 2015;17(2):495-28.
United States Census Bureau. Annual Estimates of the Resident Population by Single Year of Age and Sex for the United States, States, and Puerto Rico Commonwealth: April 1, 2010 to July 1, 2016 2016 Population Estimates. 2017 [cited 2018 Mar 14]. Available from: https://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?src=bkmk
Chen X, Yu B, Lasopa S, Cottler LB. Current patterns of marijuana use initiation by age among US adolescents and emerging adults: implications for intervention. Am J Drug Alcohol Abuse. 2017;43(3):261–70.
Miech R, Koester S. Trends in U.S., past-year marijuana use from 1985 to 2009: an age-period-cohort analysis. Drug Alcohol Depend. 2012;124(3):259–67.
Steinberg L. The influence of neuroscience on US supreme court decisions about adolescents’ criminal culpability. Nat Rev Neurosci. 2013;14(7):513–8.
Sarvet AL, Wall MM, Fink DS, Greene E, Le A, Boustead AE, et al. Medical marijuana laws and adolescent marijuana use in the United States: a systematic review and meta-analysis. Addiction. 2018;113(6):1003–16.
Hasin DS, Wall M, Keyes KM, Cerdá M, Schulenberg J, O’Malley PM, et al. Medical marijuana laws and adolescent marijuana use in the USA from 1991 to 2014: results from annual, repeated cross-sectional surveys. Lancet Psychiatry. 2015;2(7):601–8.
Pacula RL, Chriqui JF, King J. Marijuana decriminalization: what does it mean in the United States? National Bureau of Economic Research; 2003.
Donnelly N, Hall W, Christie P. The effects of the Cannabis expiation notice system on the prevalence of cannabis use in South Australia: evidence from the National Drug Strategy Household Surveys 1985-95. Drug Alcohol Rev. 2000;19(3):265–9.
Gorman DM, Huber JC. Do medical cannabis laws encourage cannabis use? Int J Drug Policy. 2007;18(3):160–7.
Lynne-Landsman SD, Livingston MD, Wagenaar AC. Effects of state medical marijuana laws on adolescent marijuana use. Am J Public Health. 2013 Aug;103(8):1500–6.
Pacula RL, Powell D, Heaton P, Sevigny EL. Assessing the effects of medical marijuana laws on marijuana and alcohol use: the devil is in the details. National Bureau of Economic Research; 2013.
Harper S, Strumpf EC, Kaufman JS. Do medical marijuana laws increase marijuana use? Replication study and extension. Ann Epidemiol. 2012;22(3):207–12.
Stolzenberg L, D’Alessio SJ, Dariano D. The effect of medical cannabis laws on juvenile cannabis use. Int J Drug Policy. 2016;27:82–8.
Wang GS, Roosevelt G, Heard K. Pediatric marijuana exposures in a medical marijuana state. JAMA Pediatr. 2013;167(7):630–3.
Wall MM, Poh E, Cerdá M, Keyes KM, Galea S, Hasin DS. Adolescent marijuana use from 2002 to 2008: higher in states with medical marijuana laws, cause still unclear. Ann Epidemiol. 2011;21(9):714–6.
Chen X, Yu B, Stanton B, Cook RL, Chen D-GD, Okafor C. Medical marijuana laws and marijuana use among U.S. adolescents: evidence from michigan youth risk behavior surveillance data. J Drug Educ. 2018;47237918803361.
Chen X. Information diffusion in the evaluation of medical marijuana laws’ impact on risk perception and use. Am J Public Health. 2016;106(12):e8.
Chen X, Yu B, Stanton B, Cook RL, Chen DG, Okafor C. Medical marijuana laws and marijuana use among US adolescents: Evidence from Michigan youth risk behavior surveillance data. J Drug Educ. 2018;48(1-2):18-35.
Yang Y, Land K. Age-Period-Cohort Analysis: New Models, Methods, and Empirical Applications. Boca Raton: Chapman and Hall/CRC; 2013.
Yu B, Chen X. Age and birth cohort-adjusted rates of suicide mortality among US male and female youths aged 10 to 19 years from 1999 to 2017. JAMA Netw Open. 2019;2(9):e1911383.
Akiba S. Epidemiological studies of Fukushima residents exposed to ionising radiation from the Fukushima Daiichi nuclear power plant prefecture--a preliminary review of current plans. J Radiol Prot. 2012;32(1):1–10.
Yang Y, Land KC. Age-period-cohort analysis of repeated cross-section surveys: fixed or random effects? Sociol Methods Res. 2008;36(3):297–326.
O’Brien R. Age-period-cohort models: approaches and analyses with aggregate data. Boca Raton: Chapman and Hall/CRC; 2014.
Book Google Scholar
Chen X, Sun Y, Li Z, Yu B, Gao G, Wang P. Historical trends in suicide risk for the residents of mainland China: APC modeling of the archived national suicide mortality rates during 1987-2012. Soc Psychiatry Psychiatr Epidemiol. 2018;54(1):99–110.
Yang Y. Social inequalities in happiness in the United States, 1972 to 2004: an age-period-cohort analysis. Am Sociol Rev. 2008;73(2):204–26.
Reither EN, Hauser RM, Yang Y. Do birth cohorts matter? Age-period-cohort analyses of the obesity epidemic in the United States. Soc Sci Med. 2009;69(10):1439–48.
Kerr WC, Lui C, Ye Y. Trends and age, period and cohort effects for marijuana use prevalence in the 1984-2015 US National Alcohol Surveys. Addiction. 2018;113(3):473–81.
Johnson RA, Gerstein DR. Age, period, and cohort effects in marijuana and alcohol incidence: United States females and males, 1961-1990. Substance Use Misuse. 2000;35(6–8):925–48.
Substance Abuse and Mental Health Services Administration. Results from the 2013 NSDUH: Summary of National Findings, SAMHSA, CBHSQ. 2014 [cited 2018 Sep 23]. Available from: https://www.samhsa.gov/data/sites/default/files/NSDUHresultsPDFWHTML2013/Web/NSDUHresults2013.htm
Bauer DJ, Hussong AM. Psychometric approaches for developing commensurate measures across independent studies: traditional and new models. Psychol Methods. 2009;14(2):101–25.
Drug Policy Alliance. A Brief History of the Drug War. 2018 [cited 2018 Sep 27]. Available from: http://www.drugpolicy.org/issues/brief-history-drug-war
NIDA. Drug testing in schools. 2017 [cited 2018 Sep 27]. Available from: https://www.drugabuse.gov/related-topics/drug-testing/faq-drug-testing-in-schools
Wikipedia contributors. Legal history of cannabis in the United States. 2015. Available from: https://en.wikipedia.org/w/index.php?title=Legal_history_of_cannabis_in_the_United_States&oldid=674767854 . Accessed 24 Oct 2017.
NORML. Marijuana law reform timeline. 2015. Available from: http://norml.org/shop/item/marijuana-law-reform-timeline . Accessed 24 Oct 2017.
Download references
Acknowledgements
Not applicable.
Author information
Authors and affiliations.
Department of Epidemiology, University of Florida, Gainesville, FL, 32608, USA
Bin Yu & Xinguang Chen
Department of Epidemiology and Health Statistics School of Health Sciences, Wuhan University, Wuhan, 430071, China
Xiangfan Chen & Hong Yan
You can also search for this author in PubMed Google Scholar
Contributions
BY designed the study, collected the data, conducted the data analysis, drafted and reviewed the manuscript; XGC designed the study and reviewed the manuscript. XFC and HY reviewed the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Correspondence to Hong Yan .
Ethics declarations
Ethics approval and consent to participate.
The study was reviewed and approved by the Institutional Review Board at the University of Florida. Data in the study were public available.
Consent for publication
All authors consented for the publication.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: table s1..
Estimated Age, Period, Cohort Effects for the Trend of Marijuana Use in Past Month among Adolescents and Emerging Adults Aged 12 to 25 Years, NSDUH, 1979-2016. Table S2. Laws at the federal and state levels related to marijuana use.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Yu, B., Chen, X., Chen, X. et al. Marijuana legalization and historical trends in marijuana use among US residents aged 12–25: results from the 1979–2016 National Survey on drug use and health. BMC Public Health 20 , 156 (2020). https://doi.org/10.1186/s12889-020-8253-4
Download citation
Received : 15 June 2019
Accepted : 21 January 2020
Published : 04 February 2020
DOI : https://doi.org/10.1186/s12889-020-8253-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Adolescents and young adults
- United States
BMC Public Health
ISSN: 1471-2458
- General enquiries: [email protected]
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on the Health Effects of Marijuana: An Evidence Review and Research Agenda. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington (DC): National Academies Press (US); 2017 Jan 12.

The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research.
- Hardcopy Version at National Academies Press
15 Challenges and Barriers in Conducting Cannabis Research
Several states have legalized cannabis for medical or recreational use since the release of the 1999 Institute of Medicine (IOM) 1 report Marijuana and Medicine: Assessing the Science Base ( IOM, 1999 ). As of October 2016, 25 states and the District of Columbia had legalized the medical use of cannabis, while 4 states and the District of Columbia had also legalized recreational cannabis use ( NCSL, 2016 ; NORML, 2016a ). 2 In November 2016, voters in California, Maine, Massachusetts, and Nevada approved ballot initiatives to legalize recreational cannabis, while voters in Arkansas, Florida, Montana, and North Dakota approved ballot initiatives to permit or expand the use of cannabis for medical purposes ( NORML, 2016b ).
Policy changes are associated with marked changes in patterns of cannabis use. In recent years, the number of U.S. adolescents and adults ages 12 and older who reported using cannabis increased by 35.0 percent and 20.0 percent for use in the past month and in the past year, respectively ( Azofeifa et al., 2016 ). Revenue from the sale and taxation of cannabis can serve as a proxy measure for cannabis use and suggests that the scope of cannabis use in the United States is considerable. For example, the total estimated value of legal cannabis sales in the United States was $5.7 billion in 2015 and $7.1 billion in 2016 ( Arcview Market Research and New Frontier Data, 2016 ). At the state level, the Colorado Department of Revenue reported that sales and excise taxes on recreational and medical cannabis sales totaled $88,239,323 in fiscal year 2015 ( CDOR, 2016a, p. 29 ), 3 and in Washington, state and local sales taxes and state business and occupation taxes on recreational and medical cannabis totaled $53,410,661 in fiscal year 2016 ( WDOR, 2016a , b ). 4
Despite these changes in state policy and the increasing prevalence of cannabis use and its implications for population health, the federal government has not legalized cannabis and continues to enforce restrictive policies and regulations on research into the health harms or benefits of cannabis products that are available to consumers in a majority of states. As a result, research on the health effects of cannabis and cannabinoids has been limited in the United States, leaving patients, health care professionals, and policy makers without the evidence they need to make sound decisions regarding the use of cannabis and cannabinoids. This lack of evidence-based information on the health effects of cannabis and cannabinoids poses a public health risk.
In order to promote research on cannabis and cannabinoids, the barriers to such research must first be identified and addressed. The committee identified several barriers to conducting basic, clinical, and population health research on cannabis and cannabinoids, including regulations and policies that restrict access to the cannabis products that are used by an increasing number of consumers and patients in state-regulated markets, funding limitations, and numerous methodological challenges. The following sections discuss these barriers in detail.
- REGULATORY AND SUPPLY BARRIERS
Regulatory Barriers
Investigators seeking to conduct research on cannabis or cannabinoids must navigate a series of review processes that may involve the National Institute on Drug Abuse (NIDA), the U.S. Food and Drug Administration (FDA), the U.S. Drug Enforcement Administration (DEA), institutional review boards, offices or departments in state government, state boards of medical examiners, the researcher's home institution, and potential funders. A brief overview of some of these review processes is discussed.
Researchers conducting clinical research on biological products such as cannabis must submit an investigational new drug (IND) application to the FDA. As a next step, the investigator may contact NIDA, an important source of research-grade cannabis, to obtain an administrative letter of authorization (LOA). An LOA describes the manufacturer's facilities, as well as the availability and pertinent characteristics of the desired cannabis product (e.g., strains, quality, strength, pharmacology, toxicology). To safeguard against the acquisition of cannabis or cannabinoids for non-research purposes, investigators must also apply for a DEA registration and site licensure before conducting studies involving cannabis or any of its cannabinoid constituents, irrespective of their pharmacologic activity. 5 The investigator must submit the IND and LOA to the FDA and the DEA for review ( FDA, 2015 ).
After submitting an IND application, researchers must wait at least 30 days before initiating research, during which period the FDA reviews the application to ensure that research participants will not be exposed to unreasonable risk ( FDA, 2016a ). If the FDA determines that the proposed research would expose study participants to unreasonable risk or that the IND application is in some other way deficient, a clinical hold postponing the research may be imposed. This hold is not lifted until and unless the sponsoring researchers have resolved the deficiencies ( FDA, 2016b ).
It is important to note that the Controlled Substances Act of 1970 classified cannabis as a Schedule I substance, the highest level of drug restriction. 6 As defined by the Act, Schedule I substances are those that (1) have a high potential for abuse; (2) have no currently accepted medical use in treatment in the United States; and (3) have a lack of accepted safety for their use under medical supervision. 7 Other substances classified in Schedule I include heroin, LSD, mescaline, hallucinogenic amphetamine derivatives, fentanyl derivatives (synthetic opioid analgesics), and gammahydroxybutyrate (GHB). 8 By contrast, Schedule II substances—though they also have a high potential for abuse and may lead to severe psychological or physical dependence—are defined as having a currently accepted medical use and can be prescribed with a controlled substance prescription ( DEA, 2006 ). 9
In some states, researchers conducting clinical research on cannabis or cannabinoid products must also apply for and receive a controlled substance certificate from a state board of medical examiners or a controlled substance registration from a department of the state government in order to conduct clinical trials or any other activity involving Schedule I substances ( Alabama Board of Medical Examiners, 2013 ; MDHSS, n.d .). Some state governments require additional approvals. For example, California requires that all trials involving Schedule I or II controlled substances be registered with and approved by the Research Advisory Panel of California ( CADOJ/OAG, 2016 ). When the necessary approvals are secured, only then can the investigator apply for a DEA registration and site licensure to conduct research on a Schedule I controlled substance (see Box 15-1 for examples of research barriers).
Illustrative Examples of the Current Research Barriers to Colorado Researchers.
Researchers conducting trials of Schedule I substances must additionally submit a research protocol to the DEA that includes details regarding the security provisions for storing and dispensing the substance. 10 Previously, nonfederally funded studies on cannabis were also required to undergo an additional review process conducted by the Public Health Service. This review process was determined to unnecessarily duplicate the FDA's IND application process in several ways and, as of June 2015, is no longer required. 11
To ensure that controlled substances obtained for research purposes will be stored and accessed in accordance with DEA security requirements, local DEA officials may perform a preregistration inspection of the facility where the proposed research will take place ( University of Colorado, 2016 ). DEA security requirements include storing cannabis in a safe, a steel cabinet, or a vault, and limiting access to the storage facility to “an absolute minimum number of specifically authorized employees. 12 The extent of the security measures required by DEA varies with the amount of cannabis being stored, 13 and among local DEA jurisdictions ( Woodworth, 2011 ). Funders must bear the costs of meeting the necessary security requirements.
Additionally, as with any human clinical trial, approval from an institutional review board must be sought. 14 Obtaining this approval confirms that an appropriate plan to protect the rights and welfare of human research subjects has been outlined in the proposed research efforts. If a study is being conducted in a clinical research center, a separate review may be required by this entity's medical or research advisory committee.
In summary, basic and clinical researchers seeking to obtain cannabis or cannabinoids from NIDA for research purposes—including efforts to determine the value of cannabis or cannabinoids for treating a medical condition or achieving a therapeutic end need—must obtain a number of approvals from a range of federal, state, or local agencies, institutions, or organizations. This process can be a daunting experience for researchers. The substantial layers of bureaucracy that emerge from cannabis's Schedule I categorization is reported to have discouraged a number of cannabis researchers from applying for grant funding or pursuing additional research efforts ( Nutt et al., 2013 ). Given the many gaps in the research of the health effects of cannabis and cannabinoids, there is a need to address these regulatory barriers so that researchers will be better able to address key public health questions about the therapeutic and adverse effects of cannabis and cannabinoid use.
CONCLUSION 15-1 There are specific regulatory barriers, including the classification of cannabis as a Schedule I substance, that impede the advancement of cannabis and cannabinoid research. 15
Barriers to Cannabis Supply
In the United States, cannabis for research purposes is available only through the NIDA Drug Supply Program ( NIDA, 2016a ). The mission of NIDA is to “advance science on the causes and consequences of drug use and addiction and to apply that knowledge to improve individual and public health,” rather than to pursue or support research into the potential therapeutic uses of cannabis or any other drugs ( NIDA, 2016b ). As a result of this emphasis, less than one-fifth of cannabinoid research funded by NIDA in fiscal year 2015 concerns the therapeutic properties of cannabinoids ( NIDA, 2016c ). 16 Because NIDA funded the majority of all the National Institutes of Health (NIH)-sponsored cannabinoid research in fiscal year 2015 ( NIDA, 2016c ), 17 its focus on the consequences of drug use and addiction constitutes an impediment to research on the potential beneficial health effects of cannabis and cannabinoids.
All of the cannabis that NIDA provides to investigators is sourced from the University of Mississippi, which is currently the sole cultivator of the plant material and has been since 1968 ( NIDA, 1998 , 2016a ). 18 In the past, the varieties of cannabis that were available to investigators through NIDA were limited in scope and were not of comparable potency to what patients could obtain at their dispensaries ( Stith and Vigil, 2016 ). Because of restrictions on production and vicissitudes in supply and demand, federally produced cannabis may have been harvested years earlier, is stored in a freezer (a process that may affect the quality of the product) ( Taschwer and Schmid, 2015 ; Thomas and Pollard, 2016 ), and often has a lower potency than cannabis sold in state-regulated markets ( Reardon, 2015 ; Stith and Vigil, 2016 ). In addition, many products available in state-regulated markets (e.g., edibles, concentrates, oils, wax, topicals) are not commonly available through federal sources ( NIDA, 2016d ). Since the products available through the federal system do not sufficiently reflect the variety of products used by consumers, research conducted using cannabis provided by NIDA may lack external validity. In July 2016, NIDA posted a formal request for information on the varieties of cannabis and cannabis products of interest to researchers ( NIDA, 2016e ). Reflecting the perceived shortcomings of cannabis and cannabis products currently provided by NIDA, a summary of the comments received in response to this request states that “the most consistent recommendation was to provide marijuana strains and products that reflect the diversity of products available in state dispensaries” ( NIDA, 2016e ).
Naturally, it is difficult for a single facility at the University of Mississippi to replicate the array and potency of products available in dispensaries across the country. It is worth noting, however, that NIDA has been increasingly responsive to the needs of clinical investigators. For example, NIDA has contracted with the University of Mississippi to produce cannabis strains with varying concentrations of Δ 9 -tetrahydrocannabinol (THC) and cannabidiol (CBD) ( NIDA, 2016d ), and NIDA has previously authorized development of cannabis extracts, tinctures, and other dosage formulations for research purposes ( Thomas and Pollard, 2016 ). As mentioned above, NIDA has sought public comment on the needs of cannabis researchers in order to inform efforts to “expand access to diverse marijuana strains and products for research purposes” ( NIDA, 2016e ). In addition, cannabis is made available to research investigators funded by NIH at no cost. 19 Finally, the DEA has adopted a new policy that increases the number of entities that may be registered under the Controlled Substances Act (CSA) to grow (manufacture) marijuana to supply legitimate researchers in the United States. 20 Under this new policy, the DEA will facilitate cannabis research by increasing the number of private entities allowed to cultivate and distribute research-grade cannabis. As of December 2016, the University of Mississippi remains the sole cultivator of cannabis provided to researchers by NIDA ( NIDA, 2016a ).
Although new plans are being made to provide a wider array of more clinically relevant cannabis products for research, at present this issue is still a significant barrier for conducting comprehensive research on the health effects of cannabis use. How the proposed changes will affect cannabis research in the future remains to be seen.
CONCLUSION 15-2 It is often difficult for researchers to gain access to the quantity, quality, and type of cannabis product necessary to address specific research questions on the health effects of cannabis use.
Funding Limitations
Funding for research is another key barrier; without adequate financial support, cannabis research will be unable to inform health care or public health practice or to keep pace with changes in cannabis policy and patterns of cannabis use. NIH is responsible for funding research across a number of health domains. In 2015, NIH spending on all cannabinoid research totaled $111,275,219 ( NIDA, 2016c ). NIDA, a member institute of NIH, has as its mission to study factors related to substance abuse and dependence and conducts research on the negative health effects and behavioral consequences associated with the abuse of cannabis and other drugs ( NIDA, 2016b ). Because cannabis was historically perceived to have only negative effects, the majority of cannabis research has been conducted under the auspices of NIDA.
In fiscal year 2015, studies supported by NIDA accounted for 59.3 percent ($66,078,314) of all NIH spending on cannabinoid research; however, only 16.5 percent ($10,923,472) of NIDA's spending on cannabinoid research supported studies investigating therapeutic properties of cannabinoids ( NIDA, 2016c ). 21 , 22 As demonstrated in Chapter 4 of this report, a growing body of evidence suggests that cannabis and cannabinoids also have therapeutic health effects. In light of these findings, a comprehensive research agenda that investigates both the potential adverse and the potential therapeutic health effects of cannabis use is needed.
However, it may be unrealistic to expect NIDA to have the resources or interest to fund this broader research agenda, which could involve investigating the health effects of cannabis use on a diverse range of conditions (e.g., metabolic syndrome, cardiovascular disease, cancer, obesity and sedentary behavior, Alzheimer's disease) that are targeted by other institutes and centers of NIH. While it is not clear how these studies might be funded, almost assuredly the changing norms and the changing legal status of cannabis will have an impact on conditions that are targeted by institutes other than NIDA, and it will become increasingly important to have a funding mechanism to better understand the comprehensive health effects of cannabis so that consumers and policy makers can respond to changing trends accordingly.
CONCLUSION 15-3 A diverse network of funders is needed to support cannabis and cannabinoid research that explores the harmful and beneficial health effects of cannabis use.
- METHODOLOGICAL CHALLENGES
Drug Delivery Challenges
Another challenge in investigating the potential health effects of cannabis and cannabinoids is the identification of a method of administering the drug that is accepted by study participants, that can be performed at most research sites, and that ensures standardized dosing. Smoking as a route of administration is particularly challenging, as some study participants may not view it as an acceptable method of drug administration, and academic medical centers or other locations where cannabis or cannabinoid research takes place may lack facilities where study participants can smoke under controlled conditions. Furthermore, variations among individuals in terms of their cannabis smoking techniques make it difficult to ensure that study participants reliably receive the targeted dose of the drug. Devices for providing a metered dose of cannabis via inhalation exist ( Eisenberg et al., 2014 ), but the FDA has not approved such devices for use. Standardized smoking techniques have also been developed ( Foltin et al., 1988 ) but can be difficult to perform correctly. These difficulties are due, in part, to differences among individuals in their tolerance of the potential psychoactive effects of the drug ( D'Souza et al., 2008 ; Ramaekers et al., 2009 ), which may prevent the receipt of equal doses by all study participants.
Researchers have also explored vaporization as a method for administering cannabis ( Abrams et al., 2007 ). Cannabinoids vaporize at lower temperatures than the temperature at which pyrolytic toxic compounds are created through combustion; as a result, levels of some carcinogenic compounds are lower in cannabis vapor than in cannabis smoke ( Eisenberg et al., 2014 ). However, there is a paucity of research on the effectiveness of these devices as a mode of drug administration. For example, data on the plasma concentrations of cannabinoids achieved through use of vaporizers exists, but they are limited ( Abrams et al., 2007 ; Zuurman et al., 2008 ). In addition, even less is known about the long-term pulmonary effects of inhaling a vaporized liquid than about the effect of inhaling plant material. As vaporizing devices proliferate and evolve, researchers may benefit from advances in their portability and usability, but they will also have to account for clinically relevant differences in the functioning and the effectiveness of an increasingly wide range of models.
To circumvent the practical and methodological challenges involved in administration of cannabis through smoking or vaporization, investigators may choose to study the health effects of orally administered dronabinol or nabilone, which offer a more controlled method of drug delivery. However, the effects generated by these isolated cannabinoids might, at least in part, be different from those produced by the use of the whole cannabis plant, which also contains CBD and other cannabinoids, as well as terpenoids and flavonoids. As a result, extrapolating from the observed health effects associated with use of an isolated cannabinoid such as dronabinol or nabilone in order to predict the health effects associated with the use of cannabis may lead to erroneous conclusions.
The Placebo Issue
The gold standard of drug development is the prospective, randomized, double-blind, placebo-controlled clinical trial. Placebo cannabis produced by solvent extraction is available from NIDA and has a potency of 0.002 percent THC by weight and 0.001 percent CBD by weight ( NIDA, 2016d ). 23 The extraction process seems to retain the terpenoids and flavonoids so that the combusted placebo material smells similar to the true cannabis, thus helping to preserve the blinding to some extent. However, the psychoactive and vasoactive effects of cannabis pose a considerable challenge for effective blinding, since study participants who feel such effects will surmise that they are receiving cannabis or cannabinoids, and not a placebo.
Strategies to promote the effectiveness of blinding exist. For example, if the cannabis being studied has a very low THC content, study participants—especially those who, through regular use of more potent cannabis strains, are inured to the psychoactive effects of cannabis with low THC content—may not notice the psychoactive effects of the cannabis and therefore be unable to reliably determine whether they are using cannabis or a placebo. There is also a possibility that cannabis products with a lower ratio of the concentration of THC to the concentration of CBD may have less psychoactivity than products with a comparatively higher ratio of the concentration of THC to the concentration of CBD ( Hindocha et al., 2015 ; Jacobs et al., 2016 ). Using these strains with diminished psychoactive effects could promote more effective blinding. Researchers may also try treating both study arms in a placebo-controlled cannabis trial with a mildly psychoactive or sedating drug, the effects of which may help to ensure that study participants are unable to determine whether they are receiving a placebo or cannabis. However, by introducing another active agent, the investigators risk obfuscating the results of their study.
A potential method for assessing the effectiveness of blinding in a cannabis trial is to ask study participants to guess whether they are receiving true cannabis or a placebo. If most or all of the participants correctly guess their assignment, it can be inferred that the blinding was ineffective. Whether or not such methods are employed, investigators risk undermining their study results. On the one hand, conducting the test carries the risk of discovering that attempts at blinding were ineffective, thereby rendering the study results invalid. On the other hand, not conducting the test may lead journal reviewers aware of the challenges of blinding in cannabis trials to assume that blinding was ineffective and to discount the study results accordingly. Thus, research to address the challenge of achieving reliably effective blinding in a cannabis trial is of marked importance.
Exposure Assessment
In order to arrive at valid and meaningful results, population studies on the health effects of cannabis require as detailed an ascertainment of exposure to cannabis as possible. However, obtaining such a detailed exposure history can be difficult. This is especially true for recreational cannabis use due to the lack of a standardized dose and the existence of diverse routes of administration, including multiple modes of inhalation ( Schauer et al., 2016 ). In addition, known pharmacological biomarkers of cannabis use may be unreliable in some circumstances, while population studies to identify novel pharmacological biomarkers of cannabis exposure are limited ( Hartman et al., 2016 ; Schwope et al., 2011 ). Furthermore, the wide variety of different cannabis strains developed through a long and ongoing process of cultivation and the associated variation in the concentration of active substances in cannabis further complicate the characterization of cannabis exposure ( ElSohly and Gul, 2014 ; Elsohly et al., 2016 ; Mehmedic et al., 2010 ). Finally, recreational cannabis may contain chemical contaminants or adulterants ( Busse et al., 2008 ). Cannabis users may be unaware of the presence of these chemicals, making it unlikely that such chemicals would be identified through toxicological evaluation unless the user became involved in a forensic investigation.
Most observational studies, particularly case-control and cohort studies, depend on self-report in order to assess cannabis exposure. These reports may be incomplete, inaccurate, or imprecise due to failure on the part of investigators to ask cannabis users detailed questions about their cannabis exposure history, including the source of their cannabis exposure (e.g., smoking, edibles, vaping), or because users themselves may have limited knowledge of some aspects of their exposure or may be resistant to reporting some information. Personal recall of substance use may also be affected by other factors. For example, memory problems have been identified as a cause of inaccuracies in reporting drug use ( Johnson and Fendrich, 2005 ; Pedersen, 1990 ). In other cases, study participants may not report illicit substance use in an attempt to conform to perceived social norms ( Johnson and Fendrich, 2005 ). Similarly, individuals with substance dependency syndromes may have psychiatric comorbidity that affects the accuracy of reporting.
Finally, important information often missing from cannabis exposure histories is the extent of other substance use. As noted in Chapter 14 , there is limited evidence that cannabis use is associated with the use of other licit or illicit substances. Despite this association and the confounding effect of polysubstance use on evaluations of the health effects of cannabis use, surveys used to characterize cannabis exposure histories do not always assess for the presence of other substance use. Since secondhand exposure to cannabis smoke can have minor health effects, there may also be value in assessing for such exposure as part of larger assessments of cannabis exposure ( Herrmann et al., 2015 ).
Cannabis-Related Study Designs
In researching the health outcomes of cannabis use, the committee identified a number of studies, particularly cohort studies, of general health outcomes such as all-cause mortality or important chronic illnesses such as cancers or cardiovascular diseases. For both cohort and case-control studies, a better assessment of cannabis use would offer more valuable information, such as years of use and age at first use. Particularly for cohort studies, this would offer better ascertainment of the duration and net burden of use as well as more insight into period and age effects. As discussed in the proceeding health outcomes chapters of the report, in many of the existing cohort studies cannabis use was often queried only at baseline, and thus there was little information on interval use over time or on the variation or cessation in that use. There was also very limited information on interval health events as the cohorts progressed, impeding a summarization of long-term use and the consequent health effects. Attention to these issues will likely improve the precision of study findings.
CONCLUSION 15-4 To develop conclusive evidence for the effects of cannabis use on short- and long-term health outcomes, improvements and standardization in research methodology (including those used in controlled trials and observational studies) are needed.
The methodological challenges and the regulatory, financial, and access barriers described above markedly affect the ability to conduct comprehensive basic, clinical, and public health research on the health effects of cannabis use, with further consequences for the many potential beneficiaries of such research. In the absence of an appropriately funded and supported cannabis research agenda, patients may be unaware of viable treatment options, providers may be unable to prescribe effective treatments, policy makers may be hindered from developing evidence-based policies, and health care organizations and insurance providers lack a basis on which to revise their care and coverage policies. In short, such barriers represent a public health problem. See Box 15-2 for a summary of the chapter conclusions.
Summary of Chapter Conclusions .
- Abrams DI, Vizoso HP, Shade SB, Jay C, Kelly ME, Benowitz NL. Vaporization as a smokeless cannabis delivery system: A pilot study. Clinical Pharmacology & Therapeutics. 2007; 82 (5):572–578. [ PubMed : 17429350 ]
- Alabama Board of Medical Examiners. Alabama Board of Medical Examiners Administrative Code. 2013. [December 29, 2016]. Chapter 540-X-4: Controlled Substances Certificate. http://www .alabamaadministrativecode .state .al.us/docs/mexam/540-X-4.pdf .
- Arcview Market Research and New Frontier Data. The State of Legal Marijuana Markets, 4th Edition: Executive Summary. San Francisco, CA: The Arcview Group; 2016. [December 8, 2016]. http://mjardin .com/wp-content /uploads/2016 /05/Executive-Summary-State-of-Legal-Marijuana-Markets-4th-Edition-1.pdf .
- Azofeifa A, Mattson ME, Schauer G, McAfee T, Grant A, Lyerla R. National estimates of marijuana use and related indicators—National Survey on Drug Use and Health, United States, 2002-2014. The Morbidity and Mortality Weekly Report Surveillance Summaries. 2016; 65 (11):1–28. [ PubMed : 27584586 ]
- Busse F, Omidi L, Timper K, Leichtle A, Windgassen M, Kluge E, Stumvoll M. Lead poisoning due to adulterated marijuana. New England Journal of Medicine. 2008; 358 (15):1641–1642. [ PubMed : 18403778 ]
- CADOJ/OAG (State of California Department of Justice/Office of the Attorney General). Research Advisory Panel: Guidelines. 2016. [November 3, 2016]. https://oag .ca.gov/research/guide .
- CDOR (Colorado Department of Revenue). Annual Report 2015. Denver: Colorado Department of Revenue; 2016a. [December 8, 2016]. https://www .colorado .gov/pacific/sites/default /files/2015%20Annual%20Report_1 .pdf .
- CDOR. MED 2015 Annual Update. Denver, CO: Colorado Department of Revenue; 2016b. [December 7, 2016]. https://www .colorado .gov/pacific/sites/default /files/2015%20Annual %20Update%20FINAL%2009262016_1.pdf .
- DEA (U.S. Drug Enforcement Administration). Practitioner's Manual: An Informational Outline of the Controlled Substances Act. Washington, DC: Drug Enforcement Administration; 2006. [December 28, 2016]. Section V: Valid Prescription Requirements; pp. 18–22. https://www .deadiversion .usdoj.gov/pubs/manuals /pract/pract_manual012508.pdf .
- D'Souza DC, Ranganathan M, Braley G, Gueorguieva R, Zimolo Z, Cooper T, Perry E, Krystal J. Blunted psychotomimetic and amnestic effects of delta-9-tetrahydrocannabinol in frequent users of cannabis. Neuropsychopharmacology. 2008; 33 (10):2505–2516. [ PMC free article : PMC3799954 ] [ PubMed : 18185500 ]
- Eisenberg E, Ogintz M, Almog S. The pharmacokinetics, efficacy, safety, and ease of use of a novel portable metered-dose cannabis inhaler in patients with chronic neuropathic pain: A phase 1a study. Journal of Pain & Palliative Care Pharmacotherapy. 2014; 28 (3):216–225. [ PubMed : 25118789 ]
- ElSohly M, Gul W. Chapter 5: The Chemical Phenotypes (Chemotypes) of Cannabis. In: Pertwee R, editor. Handbook of Cannabis. New York: Oxford University Press; 2014. pp. 89–110.
- ElSohly MA, Mehmedic Z, Foster S, Gon C, Chandra S, Church JC. Changes in cannabis potency over the last 2 decades (1995-2014): Analysis of current data in the United States. Biological Psychiatry. 2016; 79 (7):613–619. [ PMC free article : PMC4987131 ] [ PubMed : 26903403 ]
- FDA (U.S. Food and Drug Administration). Marijuana research with human subjects. 2015. [January 3, 2017]. http://www .fda.gov/NewsEvents /PublicHealthFocus/ucm421173 .htm .
- FDA. Investigational New Drug (IND) Application: Introduction. 2016a. [December 8, 2016]. http://www .fda.gov/drugs /developmentapprovalprocess /howdrugsaredevelopedandapproved /approvalapplications /investigationalnewdrugindapplication/default.htm .
- FDA. IND application procedures: Clinical hold. 2016b. [December 8, 2016]. http://www .fda.gov/Drugs /DevelopmentApprovalProcess /HowDrugsareDevelopedandApproved /ApprovalApplications /InvestigationalNewDrugINDApplication/ucm362971.htm .
- Foltin R, Fischman M, Byrne M. Effects of smoked marijuana on food intake and body weight of humans living in a residential laboratory. Appetite. 1988; 25 :577–582. [ PubMed : 3228283 ]
- Hartman RL, Brown TL, Milavetz G, Spurgin A, Gorelick DA, Gaffney GR, Huestis MA. Effect of blood collection time on measured delta9-tetrahydrocannabinol concentrations: Implications for driving interpretation and drug policy. Clinical Chemistry. 2016; 62 (2):367–377. [ PubMed : 26823611 ]
- Herrmann ES, Cone EJ, Mitchell JM, Bigelow GE, LoDico C, Flegel R, Vandrey R. Non-smoker exposure to secondhand cannabis smoke II: Effect of room ventilation on the physiological, subjective, and behavioral/cognitive effects. Drug and Alcohol Dependence. 2015; 151 :194–202. [ PMC free article : PMC4747424 ] [ PubMed : 25957157 ]
- Hindocha C, Freeman TP, Schafer G, Gardener C, Das RK, Morgan CJ, Curran HV. Acute effects of delta-9-tetrahydrocannabinol, cannabidiol and their combination on facial emotion recognition: A randomised, double-blind, placebo-controlled study in cannabis users. European Neuropsychopharmacology. 2015; 25 (3):325–334. [ PMC free article : PMC4398332 ] [ PubMed : 25534187 ]
- IOM (Institute of Medicine). Marijuana and medicine: Assessing the science base. Washington, DC: National Academy Press; 1999. [ PubMed : 25101425 ]
- Jacobs DS, Kohut SJ, Jiang S, Nikas SP, Makriyannis A, Bergman J. Acute and chronic effects of cannabidiol on delta(9)-tetrahydrocannabinol (delta(9)-THC)induced disruption in stop signal task performance. Experimental and Clinical Psychopharmacology. 2016; 24 (5):320–330. [ PMC free article : PMC5119678 ] [ PubMed : 27690502 ]
- Johnson T, Fendrich M. Modeling sources of self-report bias in a survey of drug use epidemiology. Annals of Epidemiology. 2005; 15 (5):381–389. [ PubMed : 15840552 ]
- Marijuana Business Daily Staff. Marijuana Business Daily. Jun 13, 2016. [December 29, 2016]. Chart of the Week: Sales of Marijuana Concentrates, Edibles Surging in Colorado. http://mjbizdaily .com /chart-of-the-week-sales-of-marijuana-concentrates-edibles-surging-in-colorado .
- Mehmedic Z, Chandra S, Slade D, Denham H, Foster S, Patel AS, Ross SA, Khan IA, ElSohly MA. Potency trends of delta9-THC and other cannabinoids in confiscated cannabis preparations from 1993 to 2008. Journal of Forensic Science. 2010; 55 (5):1209–1217. [ PubMed : 20487147 ]
- MDHSS (Missouri Department of Health and Senior Services). Frequently Asked Questions. n.d. [December 29, 2016]. http://health .mo.gov/safety/bndd/faqs .php .
- NCSL (National Conference of State Legislatures). State Medical Marijuana Laws. 2016. [December 22, 2016]. http://www .ncsl.org/research /health/state-medical-marijuana-laws.aspx .
- NIDA (National Institute on Drug Abuse). Provision of Marijuana and Other Compounds for Scientific Research—Recommendations of the National Institute on Drug Abuse National Advisory Council. 1998. [December 29, 2016]. https://archives .drugabuse .gov/about/organization /nacda/MarijuanaStatement.html .
- NIDA. Information on Marijuana Farm Contract. 2015. [December 29, 2016]. https://www .drugabuse .gov/drugs-abuse/marijuana /nidas-role-in-providing-marijuana-research /informationmarijuana-farm-contract .
- NIDA. NIDA's Role in Providing Marijuana for Research. 2016a. [December 8, 2016]. https://www .drugabuse .gov/drugs-abuse/marijuana /nidas-role-in-providing-marijuana-research .
- NIDA. National Institute on Drug Abuse (NIDA): Mission. 2016b. [December 9, 2016]. https://www .nih.gov/about-nih /what-we-do /nih-almanac/national-institute-drug-abuse-nida .
- NIDA. NIH Research on Marijuana and Cannabinoids. 2016c. [December 29, 2016]. https://www .drugabuse .gov/drugs-abuse/marijuana /nih-research-marijuana-cannabinoids .
- NIDA. Marijuana plant material available from the NIDA drug supply program. 2016d. [November 3, 2016]. https://www .drugabuse .gov/researchers/research-resources /nida-drug-supplyprogram-dsp /marijuana-plant-material-available-nida-drug-supply-program .
- NIDA. Summary of Request for Information (RFI) Regarding Varieties of Marijuana and Marijuana Products for Research. 2016e. [November 3, 2016]. https://www .drugabuse .gov/drugsabuse/marijuana /nidas-role-in-providing-marijuana-research /summary-requestinformation-rfi-regarding-varieties-marijuana-marijuana-products-research .
- NIH (National Institutes of Health). Estimates of Funding for Various Research, Condition, and Disease Categories (RCDC). 2016. [December 29, 2016]. https://report .nih.gov /categorical_spending.aspx#legend1 .
- NORML (National Organization for the Reform of Marijuana Laws). About Marijuana. 2016a. [December 22, 2016]. http://norml .org/marijuana .
- NORML. Election 2016—Marijuana Ballot Results. 2016b. [December 22, 2016]. http://norml .org/election-2016 .
- Nutt DJ, King LA, Nichols DE. Effects of Schedule I drug laws on neuroscience research and treatment innovation. Nature Reviews Neuroscience. 2013; 14 (8):577–585. [ PubMed : 23756634 ]
- Pedersen W. Reliability of drug use responses in a longitudinal study. Scandinavian Journal of Psychology. 1990; 31 (1):28–33. [ PubMed : 2333484 ]
- Ramaekers JG, Kauert G, Theunissen EL, Toennes SW, Moeller MR. Neurocognitive performance during acute thc intoxication in heavy and occasional cannabis users. Journal of Psychopharmacology. 2009; 23 (3):266–277. [ PubMed : 18719045 ]
- Reardon S. Marijuana gears up for production high in U.S. labs. Nature. 2015; 519 (7543):269–270. [ PubMed : 25788072 ]
- Schauer GL, King BA, Bunnell RE, Promoff G, McAfee TA. Toking, vaping, and eating for health or fun: Marijuana use patterns in adults, U.S., 2014. American Journal of Preventative Medicine. 2016; 50 (1):1–8. [ PubMed : 26277652 ]
- Schwope DM, Karschner EL, Gorelick DA, Huestis MA. Identification of recent cannabis use: Whole-blood and plasma free and glucuronidated cannabinoid pharmacokinetics following controlled smoked cannabis administration. Clinical Chemistry. 2011; 57 (10):1406–1414. [ PMC free article : PMC3717336 ] [ PubMed : 21836075 ]
- Stith SS, Vigil JM. Federal barriers to cannabis research. Science. 2016; 352 (6290):1182. [ PubMed : 27257247 ]
- Taschwer M, Schmid MG. Determination of the relative percentage distribution of THCA and D(9)-THC in herbal cannabis seized in Austria—Impact of different storage temperatures on stability. Forensic Science International. 2015; 254 :167–171. [ PubMed : 26247127 ]
- Thomas BF, Pollard GT. Preparation and distribution of cannabis and cannabis-derived dosage formulations for investigational and therapeutic use in the United States. Frontiers in Pharmacology. 2016; 7 :285. [ PMC free article : PMC5006560 ] [ PubMed : 27630566 ]
- University of Colorado. Drug Enforcement Administration (DEA) Controlled Substances. 2016. [December 22, 2016]. http://www .ucdenver.edu /research/EHS/hazmat/Pages/DEA.aspx .
- WDOR (Washington Department of Revenue). Medical Marijuana Taxes. 2016a. [December 8, 2016]. http://dor .wa.gov/Docs /Reports/2014/MMJTax.xlsx .
- WDOR. Recreational Marijuana Taxes. 2016b. [December 8, 2016]. http://dor .wa.gov/Docs /Reports/2014/RMJTax.xlsx .
- Woodworth TW. How will DEA affect your clinical study? Journal of Clinical Research Best Practices. 2011; 7 (12) [December 8, 2016]; https: //firstclinical .com/journal/2011/1112_DEA.pdf .
- Zuurman L, Roy C, Schoemaker RC, Hazekamp A, den Hartigh J, Bender JC, Verpoorte R, Pinquier JL, Cohen AF, van Gerven JM. Effect of intrapulmonary tetrahydrocannabinol administration in humans. Journal of Psychopharmacology. 2008; 22 (7):707–716. [ PubMed : 18515447 ]
As of March 2016, the Health and Medicine Division continues the task of producing consensus studies and convening activities previously undertaken by the Institute of Medicine (IOM).
The count of states where cannabis is legalized for medical use includes Ohio and Pennsylvania, where medical cannabis laws were not operational as of October 2016 ( NCSL, 2016 ).
$22,225,750 (Marijuana Sales Tax [2.9%]) + $42,017,798 (Retail Marijuana Sales Tax [10%]) + $23,995,775 (Retail Marijuana Excise Tax [15%]) = $88,239,323.
Medical Cannabis: $5,236,536 (State Retail Sales Tax) + $792,906 (State Business and Occupation Tax) + $ 2,084,323 (Local Retail Sales Tax) = $8,113,765. Recreational Cannabis: $30,017,823 (State Retail Sales Tax) + $4,050,212 (State Business & Occupation Tax) + $11,228,861 (Local Retail Sales Tax) = $45,296,896. $8,113,765 (Total Medical Cannabis Taxes) + $45,296,896 (Total Recreational Cannabis Taxes) = $53,410,661.
Code of Federal Regulations, Registration of Manufacturers, Distributors, and Dispensers of Controlled Substances, Title 21, § 1301.11 and Code of Federal Regulations, Schedules of Controlled Substances, Title 21, § 1308.11.
Code of Federal Regulations, Schedules of Controlled Substances, Title 21, § 1308.11; United States Code, Schedules of Controlled Substances, Title 21, § 812.
United States Code, Schedules of Controlled Substances, Title 21, § 812(b)(1).
Code of Federal Regulations, Schedules of Controlled Substances, Title 21, § 1308.11.
United States Code, Schedules of Controlled Substances, Title 21, § 812(b)(2).
Code of Federal Regulations, Registration of Manufacturers, Distributors, and Dispensers of Controlled Substances, Title 21, § 1301.18.
Office of the Secretary, Office of the Assistant Secretary for Health, U.S. Department of Health and Human Services. Notice. “Announcement of Revision to the Department of Health and Human Services Guidance on Procedures for the Provision of Marijuana for Medical Research as Published on May 21, 1999,” Federal Register , 80, no. 120 (June 23, 2015): 35960, https://www .gpo.gov/fdsys /pkg/FR-2015-06-23/pdf/2015-15479 .pdf (accessed November 25, 2016).
Code of Federal Regulations, Registration of Manufacturers, Distributors, and Dispensers of Controlled Substances, Title 21, § 1301.72 (a) and (d).
Code of Federal Regulations, Registration of Manufacturers, Distributors, and Dispensers of Controlled Substances, Title 21, § 1301.71 (c).
Code of Federal Regulations, Institutional Review Boards, Title 21, § 56.103.
The committee was specifically directed in its statement of task not to comment on cannabis policy issues, such as regulatory options for legalization, taxation, or distribution. While the committee has identified the Schedule 1 classification of cannabis as posing a significant barrier to the conduct of scientific research on the health effects of cannabis, the committee is aware that any decision on the regulation of cannabis involves many factors far outside the committee's remit and expertise. Specifically, the committee did not comment on the abuse or dependency liability or accepted medical use of cannabis compared to other scheduled drugs.
In fiscal year 2015, NIDA's investment in cannabinoid research totaled $66,078,314, of which $10,923,472 was allocated for therapeutic cannabinoid research ( NIDA, 2016c ).
In fiscal year 2015, NIH's investment in cannabinoid research totaled $ $111,275,219, of which $66,078,314 was allocated to NIDA ( NIDA, 2016c ).
NIDA contracts with the University of Mississippi through an open solicitation process. Although the University of Mississippi is currently NIDA's only supplier of research-grade cannabis, other groups can compete for the contract ( NIDA, 2015 , 2016a ).
In December 2016, cannabis provided by NIDA was generally free for NIH-sponsored research. For research not funded by the federal government, the cost of non-placebo cannabis was $10.96 per cigarette and $1,133 per pound ($2,497 per kilogram) ( NIDA, 2016d ).
DEA, U.S. Department of Justice. Policy Statement. “Applications to Become Registered Under the Controlled Substances Act to Manufacture Marijuana to Supply Researchers in the United States,” Federal Register , 81, no. 156 (August 12, 2016): 53846, https://www .gpo.gov/fdsys /pkg/FR-2016-08-12/pdf/2016-17955 .pdf (accessed January 7, 2017).
$66,078,314 (Total NIDA spending on cannabinoid research in fiscal year 2015)/$111,275,219 (Total NIH spending on cannabinoid research in fiscal year 2015) = 0.593. $10,923,472 (Total NIDA spending on therapeutic cannabinoid research in fiscal year 2015)/$66,078,314 (Total NIDA spending on cannabinoid research in fiscal year 2015) = 0.165.
By contrast, NIH spending on tobacco research totaled $300 million in 2015, and spending on research related to the harms and benefits of alcohol use totaled $473 million in 2015 ( NIH, 2016 ).
In December 2016, placebo cannabis provided by NIDA was generally free for NIH-sponsored research. For research not funded by the federal government, the cost of placebo cannabis was $13.94 per cigarette and $1,133 per pound ($2,497 per kilogram) ( NIDA, 2016d ).
- Cite this Page National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on the Health Effects of Marijuana: An Evidence Review and Research Agenda. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington (DC): National Academies Press (US); 2017 Jan 12. 15, Challenges and Barriers in Conducting Cannabis Research.
- PDF version of this title (3.3M)
In this Page
Other titles in this collection.
- The National Academies Collection: Reports funded by National Institutes of Health
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Recent Activity
- Challenges and Barriers in Conducting Cannabis Research - The Health Effects of ... Challenges and Barriers in Conducting Cannabis Research - The Health Effects of Cannabis and Cannabinoids
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers

IMAGES
VIDEO
COMMENTS
Maternal marijuana exposure and birth weight: an observational study surrounding recreational marijuana legalization. Am J Perinatol. 2021;38:65-75. [Google Scholar] 91. Rusby JC, Westling E, Crowley R, Mills KL, Light JM. Associations between marijuana use and anxious mood lability during adolescence. Addict Behav. 2019;92:89-94.
Weed Research publishes topical and innovative papers on all aspects of weeds - weeds being defined as plants that adversely impact the economic, aesthetic, or environmental aspects of a system.. Our topics include weed biology and ecology, integrated weed management, herbicide resistance, invasive species, genetics and genomics, and novel weed control technology.
Journal of Cannabis Research is a broad scope open access journal sponsored by the Institute of Cannabis Research at Colorado State University-Pueblo (USA). ... Skip to main content. ... Marijuana use is increasingly common among patients with chronic non-cancer pain (CNCP) and long-term opioid therapy (LTOT). ... Source Normalized Impact per ...
For example, there is some evidence to suggest that in patients with symptoms of human immunodeficiency virus (HIV) infection or AIDS, marijuana use may actually exacerbate HIV-associated cognitive deficits. 75 Similarly, more research is needed to understand the potential effects of marijuana use on age-related cognitive decline in general and ...
This paper presents the up-to-date reported investigations and opens many reflections and further research perspectives. Keywords: Cannabis sativa L., ethnobotany, chemical composition, biological activities, medicine, in silico, molecular docking. 1. Introduction. Cannabis sativa L. is an herbaceous plant belonging to the Cannabaceae family ...
The Journal of Cannabis Research is the official publication of the Institute of Cannabis Research (ICR), which was established in June 2016 through an innovative partnership between Colorado State University Pueblo, the state of Colorado, and Pueblo County.. The ICR is the first US multi-disciplinary cannabis research center at a regional, comprehensive institution.
Marijuana is the most commonly used illicit drug in the United States. More and more states legalized medical and recreational marijuana use. Adolescents and emerging adults are at high risk for marijuana use. This ecological study aims to examine historical trends in marijuana use among youth along with marijuana legalization. Data (n = 749,152) were from the 31-wave National Survey on Drug ...
Several states have legalized cannabis for medical or recreational use since the release of the 1999 Institute of Medicine (IOM)1 report Marijuana and Medicine: Assessing the Science Base (IOM, 1999). As of October 2016, 25 states and the District of Columbia had legalized the medical use of cannabis, while 4 states and the District of Columbia had also legalized recreational cannabis use ...
National Bureau of Economic Research. NBER working papers are circulated for discussion and comment purposes. They have not been ... marijuana appearing in economics journals and leading public policy, public health, and medical journals during the period 2013-2020. Only 4 articles on this topic were published in 2013.
Importance With rising rates of marijuana use in the general population and an increasing number of states legalizing recreational marijuana use and authorizing medical marijuana programs, there ...