
Along with Stanford news and stories, show me:
- Student information
- Faculty/Staff information
We want to provide announcements, events, leadership messages and resources that are relevant to you. Your selection is stored in a browser cookie which you can remove at any time using “Clear all personalization” below.
A new, nano-scale look at how the SARS-CoV-2 virus replicates in cells may offer greater precision in drug development, a Stanford University team reports in Nature Communications . Using advanced microscopy techniques, the researchers produced what might be some of the most crisp images available of the virus’s RNA and replication structures, which they witnessed form spherical shapes around the nucleus of the infected cell.
“We have not seen COVID infecting cells at this high resolution and known what we are looking at before,” said Stanley Qi , Stanford associate professor of bioengineering in the Schools of Engineering and of Medicine and co-senior author of the paper. “Being able to know what you are looking at with this high resolution over time is fundamentally helpful to virology and future virus research, including antiviral drug development.”
Blinking RNA
The work illuminates molecular-scale details of the virus’ activity inside host cells. In order to spread, viruses essentially take over cells and transform them into virus-producing factories, complete with special replication organelles. Within this factory, the viral RNA needs to duplicate itself over and over until enough genetic material is gathered up to move out and infect new cells and start the process over again.
The Stanford scientists sought to reveal this replication step in the sharpest detail to date. To do so, they first labeled the viral RNA and replication-associated proteins with fluorescent molecules of different colors. But imaging glowing RNA alone would result in fuzzy blobs in a conventional microscope. So they added a chemical that temporarily suppresses the fluorescence. The molecules would then blink back on at random times, and only a few lit up at a time. That made it easier to pinpoint the flashes, revealing the locations of the individual molecules.
Using a setup that included lasers, powerful microscopes, and a camera snapping photos every 10 milliseconds, the researchers gathered snapshots of the blinking molecules. When they combined sets of these images, they were able to create finely detailed photos showing the viral RNA and replication structures in the cells. “We have highly sensitive and specific methods and also high resolution,” said Leonid Andronov, co-lead author and Stanford chemistry postdoctoral scholar. “You can see one viral molecule inside the cell.”
The resulting images, with a resolution of 10 nanometers, reveal what might be the most detailed view yet of how the virus replicates itself inside of a cell. The images show magenta RNA forming clumps around the nucleus of the cell, which accumulate into a large repeating pattern. “We are the first to find that viral genomic RNA forms distinct globular structures at high resolution,” said Mengting Han, co-lead author and Stanford bioengineering postdoctoral scholar.
Video showing the different colored fluorescent labels blinking on and off, revealing more precise locations for individual molecules. | Leonid Andronov, Moerner Laboratory
The clusters help show how the virus evades the cell’s defenses, said W. E. Moerner , the paper’s co-senior author and Harry S. Mosher Professor of Chemistry in the School of Humanities and Sciences. “They’re collected together inside a membrane that sequesters them from the rest of the cell, so that they’re not attacked by the rest of the cell.”
Nanoscale drug testing
Compared to using an electron microscope, the new imaging technique can allow researchers to know with greater certainty where virus components are in a cell thanks to the blinking fluorescent labels. It also can provide nanoscale details of cell processes that are invisible in medical research conducted through biochemical assays. The conventional techniques “are completely different from these spatial recordings of where the objects actually are in the cell, down to this much higher resolution,” said Moerner. “We have an advantage based on the fluorescent labeling because we know where our light is coming from.”
Seeing exactly how the virus stages its infection holds promise for medicine. Observing how different viruses take over cells may help answer questions such as why some pathogens produce mild symptoms while others are life-threatening. The super-resolution microscopy can also benefit drug development. “This nanoscale structure of the replication organelles can provide some new therapeutic targets for us,” said Han. “We can use this method to screen different drugs and see its influence on the nanoscale structure.”
Indeed, that’s what the team plans to do. They will repeat the experiment and see how the viral structures shift in the presence of drugs like Paxlovid or remdesivir. If a candidate drug can suppress the viral replication step, that suggests the drug is effective at inhibiting the pathogen and making it easier for the host to fight the infection.
The researchers also plan to map all 29 proteins that make up SARS-CoV-2 and see what those proteins do across the span of an infection. “We hope that we will be prepared to really use these methods for the next challenge to quickly see what’s going on inside and better understand it,” said Qi.
For more information
Acknowledgements: Additional Stanford co-authors include postdoctoral scholar Yanyu Zhu, PhD student Ashwin Balaji, former PhD student Anish Roy, postdoctoral scholar Andrew Barentine, research specialist Puja Patel, and Jaishree Garhyan, director of the In Vitro Biosafety Level-3 Service Center . Moerner is also a member of Stanford Bio-X and the Wu Tsai Neurosciences Institute, and a faculty fellow of Sarafan ChEM-H . Qi is also a member of Bio-X, the Cardiovascular Institute , the Maternal & Child Health Research Institute (MCHRI), the Stanford Cancer Institute, and the Wu Tsai Neurosciences Institute, an institute scholar at Sarafan ChEM-H , and a Chan Zuckerberg Biohub – San Francisco Investigator.
This research was funded by the National Institute of General Medical Sciences of the National Institutes of Health. We also acknowledge use of the Stanford University Cell Sciences Imaging Core Facility.
Taylor Kubota, Stanford University: [email protected]
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
Coordinating virus research: The Virus Infectious Disease Ontology
Roles Conceptualization, Formal analysis, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliations Department of Philosophy, University at Buffalo, Buffalo, NY, United States of America, National Center for Ontological Research, Buffalo, NY, United States of America
Roles Conceptualization, Investigation, Validation, Writing – review & editing
Affiliations National Center for Ontological Research, Buffalo, NY, United States of America, Air Force Research Laboratory, Wright Patterson Air Force Base, Riverside, OH, United States of America
Roles Conceptualization, Investigation, Methodology, Project administration, Validation, Writing – review & editing
Affiliation Department of Cognitive Science, Northwestern University, Evanston, IL, United States of America
Roles Conceptualization, Formal analysis, Investigation, Writing – review & editing
Affiliation Department of Clinical Sciences, University of Texas Southwestern Medical Center, Dallas, TX, United States of America
Roles Conceptualization, Formal analysis, Writing – review & editing
Affiliation Department of Philosophy, Loyola University, Chicago, IL, United States of America
Affiliation Computational Medicine and Bioinformatics, University of Michigan Medical School, He Group, Ann Arbor, MI, United States of America
Roles Conceptualization, Investigation, Methodology, Writing – review & editing
Affiliations National Center for Ontological Research, Buffalo, NY, United States of America, Department of Philosophy, Northwestern University, Evanston, IL, United States of America
Roles Conceptualization, Investigation, Methodology, Validation, Writing – review & editing
Roles Conceptualization, Methodology, Validation, Writing – review & editing
Affiliations Department of Informatics, J. Craig Venter Institute, La Jolla, CA, United States of America, Department of Pathology, University of California, San Diego, CA, United States of America, Division of Vaccine Discovery, La Jolla Institute for Immunology, La Jolla, CA, United States of America
Roles Conceptualization, Formal analysis, Investigation, Methodology, Supervision, Writing – review & editing
- John Beverley,
- Shane Babcock,
- Gustavo Carvalho,
- Lindsay G. Cowell,
- Sebastian Duesing,
- Yongqun He,
- Regina Hurley,
- Eric Merrell,
- Richard H. Scheuermann,
- Barry Smith

- Published: January 18, 2024
- https://doi.org/10.1371/journal.pone.0285093
- Peer Review
- Reader Comments
The COVID-19 pandemic prompted immense work on the investigation of the SARS-CoV-2 virus. Rapid, accurate, and consistent interpretation of generated data is thereby of fundamental concern. Ontologies–structured, controlled, vocabularies–are designed to support consistency of interpretation, and thereby to prevent the development of data silos. This paper describes how ontologies are serving this purpose in the COVID-19 research domain, by following principles of the Open Biological and Biomedical Ontology (OBO) Foundry and by reusing existing ontologies such as the Infectious Disease Ontology (IDO) Core, which provides terminological content common to investigations of all infectious diseases. We report here on the development of an IDO extension, the Virus Infectious Disease Ontology (VIDO), a reference ontology covering viral infectious diseases. We motivate term and definition choices, showcase reuse of terms from existing OBO ontologies, illustrate how ontological decisions were motivated by relevant life science research, and connect VIDO to the Coronavirus Infectious Disease Ontology (CIDO). We next use terms from these ontologies to annotate selections from life science research on SARS-CoV-2, highlighting how ontologies employing a common upper-level vocabulary may be seamlessly interwoven. Finally, we outline future work, including bacteria and fungus infectious disease reference ontologies currently under development, then cite uses of VIDO and CIDO in host-pathogen data analytics, electronic health record annotation, and ontology conflict-resolution projects.
Citation: Beverley J, Babcock S, Carvalho G, Cowell LG, Duesing S, He Y, et al. (2024) Coordinating virus research: The Virus Infectious Disease Ontology. PLoS ONE 19(1): e0285093. https://doi.org/10.1371/journal.pone.0285093
Editor: Barry L. Bentley, Cardiff Metropolitan University, UNITED KINGDOM
Received: November 28, 2022; Accepted: April 12, 2023; Published: January 18, 2024
Copyright: © 2024 Beverley et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: The Virus Infectious Disease Ontology artifact can be found in the following Github repository: https://github.com/infectious-disease-ontology-extensions/ido-virus . The Coronavirus Infectious Disease Ontology artifact can be found in the following Github repository: https://github.com/CIDO-ontology/cido .
Funding: Sources of funding for this article for John Beverley and Shane Babcock stem from the NIH / NLM T5 Biomedical Informatics and Data Science Research Training Programs. Barry Smith’s source of funding stemmed from the NIH under NCATS 1UL1TR001412 (Buffalo Clinical and Translational Research Center). No other co-authors were funded to pursue work on this project. Moreover, the funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Introduction
The value of cross-discipline meta-data analysis has been evident in the COVID-19 pandemic. Early in the pandemic, for example, prostate oncologists [ 1 , 2 ] attempted to leverage existing research on enzymes crucial in host cell penetration by SARS-CoV-2 to explain differences in disease severity across sex [ 3 , 4 ]; immunologists combined insights from research on SARS-CoV-1 and MERS-CoV with chemical compound profiles to identify treatment options for SARS-CoV-2 [ 5 – 7 ]; pediatric researchers, observing that children have fewer nasal epithelia susceptible to SARS-CoV-2 infection than adults, suggested this difference may explain symptom disparities between the two groups [ 8 , 9 ]. The sheer volume of data collected by life-science researchers, the speed at which it is generated, range of its sources, quality, accuracy, and urgency of need for assessment of usefulness, has resulted in complex, multidimensional datasets, often annotated using discipline- or institution-specific terminologies and coding systems that lead to data silos [ 10 – 12 ].
Data silos emerge in life science research when data concerning an area of research is stored in a manner that makes it accessible to one group, but inaccessible to others. The use of proprietary information systems, differing storage methods, and distinct coding standards across life science that is characteristic of such silos undermines interoperability, meta-data analysis, pattern identification, and discovery across disciplines [ 13 , 14 ]. Ontologies–interoperable, logically well-defined, controlled vocabularies representing common entities and relations across disciplines using consensus terminologies–constitute a well-known solution to these problems through mitigation of the formation of data silos. The need for rapid analysis of evolving datasets representing coronavirus research motivated the development of the Virus Infectious Disease Ontology (VIDO; https://bioportal.bioontology.org/ontologies/VIDO ), comprised of textual definitions for terms and relations and logical axioms supporting automated consistency checking, querying over datasets, and interoperability with other ontologies. VIDO is an extension of the widely-used Infectious Disease Ontology Core (IDO Core; https://bioportal.bioontology.org/ontologies/IDO ) [ 15 , 16 ], which comprises terminological content common to all investigations of infectious disease. VIDO extends IDO with terms specific to the domain of infectious diseases caused by viruses and provides a foundation for ontologies representing specific viral infectious diseases, such as COVID-19.
VIDO is available under the Creative Commons Attribution 4.0 license ( https://creativecommons.org/licenses/by/4.0/ ) and its current and past versions can be found at the National Center for Biomedical Ontology (NCBO) Bioportal [ 17 ], the Ontobee repository ( http://www.ontobee.org/ ), and the Ontology Lookup Service ( https://www.ebi.ac.uk/ols/index ). VIDO was developed in collaboration with relevant domain experts, including immunologists and virologists, and by drawing on the expertise of the IDO developers to ensure alignment with principles outlined by the Open Biological and Biomedical Ontology (OBO) Foundry [ 18 ], thereby supporting interoperability with existing Foundry ontologies [ 19 ]. VIDO development is a transparent process, with all discussions available on GitHub ( https://github.com/infectious-disease-ontology-extensions ). All aspects of development, including addition of new terms, are driven by the needs of researchers investigating viruses and nearby domains. The ontology is thus not viewed as exhaustive of the domain of virus research but remains sensitive to evolving knowledge.
OWL, Protégé, Mace4, and Prover9
VIDO is formally represented in the OWL2 Web Ontology Language ( https://www.w3.org/TR/owl2-overview/ ). OWL2 is an expansion of the Resource Description Framework (RDF; https://www.w3.org/TR/rdf-primer/ ) and of RDF Schema, which represent data as sets of subject- predicate-object directed graphs, and which can be queried using the SPARQL Protocol and RDF Query Language ( https://www.w3.org/TR/sparql11-query/ ). OWL2 supplements these languages by allowing for description of classes, members of classes, relations among individuals, and annotation properties. Formally, the OWL2 vocabulary can be mapped to a decidable fragment of first-order logic, meaning there is an algorithm which can determine the truth-value for any statement expressed in the language in a finite number of steps [ 20 ]. Restricting expressions to a decidable language allows automated consistency and satisfiability checking [ 21 ]. VIDO was developed using the Protégé-OWL editor ( https://protege.stanford.edu/ ) and tested against OWL reasoners such as HermiT [ 22 ] and Pellet [ 23 ]. Additionally, logical axioms underwriting these ontologies were translated into the Common Logic Interchange Format, and subsequently evaluated using the Mace4 model checker and Prover9 proof generator within the Macleod toolkit ( https://github.com/thahmann/macleod ).
Alignment with OBO Foundry ontologies
Ontologies are widely used in bioinformatics, supporting data standardization, integration, sharing, reproducibility, and automated reasoning. The Gene Ontology (GO; https://bioportal.bioontology.org/ontologies/GO ), for example, maintains species-neutral annotations of gene products and functions, and since its inception in 1998 it has inspired an explosion of biomedical ontologies covering all domains of the life sciences [ 19 , 24 , 25 ]. These early developments led to worries, however, that data silos–the very problem ontologies were designed to address–might reemerge [ 10 ] as researchers developed ontologies using concepts local to their discipline. By 2007, the Open Biomedical and Biological Ontologies (OBO) Foundry [ 18 ] was created to provide guidance for ontology developers and promote alignment and interoperability. OBO Foundry design principles require that ontologies: use a well-specified syntax that is unambiguous, with a common space of identifiers; that they be openly available in the public domain, have a specified scope, be developed in a modular fashion in a collaboration with ontologists covering nearby domains, and import a common set of relations from the Relations Ontology (RO; https://obofoundry.org/ontology/ro.html ). The OBO library ( http://obofoundry.org/ ) presently consists of over 250 ontologies, including some externally developed ontologies such as the NCI Thesaurus ( https://ncithesaurus.nci.nih.gov/ncitbrowser/ ) and the NCBI Taxonomy ( https://www.ncbi.nlm.nih.gov/taxonomy ). It also contains some constructed ab initio to satisfy OBO principles. At its core is Basic Formal Ontology (BFO; https://bioportal.bioontology.org/ontologies/BFO ), a top-level ontology covering general classes such as material entity , quality , process , function and role [ 10 , 26 – 29 ] which provides the architecture “on which OBO Foundry ontologies are built.” BFO is, moreover, an ISO/IEC approved standard 21838–2 ( https://www.iso.org/standard/74572.html ).
Where BFO is domain-neutral, other OBO Foundry ontologies represent types of entities in more specific domains, using terms such as disease , cell division , surgical procedure , and so forth. Ideally, domain ontologies are constructed using a methodology for formulating definitions through a process of downward population from BFO. The resulting alignment with BFO, and the conformance to OBO Foundry principles, foster integration across ontologies. VIDO was designed with alignment and conformance in mind. Development of each ontology follows metadata conventions adopted by many OBO Foundry ontologies [ 30 ]. These conventions require that every term introduced into the ontology has a unique IRI, textual definitions, definition source, designation of term editor(s), and preferred term label. In the interest of coordinating development with existing OBO ontologies, VIDO developers imported terms where possible from existing OBO library ontologies and constructed logical definitions using imported terms. Development was guided by best practices for definition construction [ 10 , 31 ]. New primitive terms were introduced when needed after consultation with domain experts, review of relevant literature, and careful examination of the OBO library to avoid redundancy.
Hub and spokes approach
VIDO follows the “hub and spokes” methodology [ 32 , 33 ] for ontology development. That is, VIDO is a spoke ontology, extending from the Infectious Disease Ontology Core (IDO Core; https://bioportal.bioontology.org/ontologies/IDO ) as its hub. IDO Core is an OBO ontology consisting of terms, relations, natural language definitions and associated logical axioms representing phenomena common across research in infectious diseases [ 15 ]. IDO Core has long provided a base from which more specific infectious disease ontologies extend, and it has been recently updated to keep pace with scientific and top-level architecture changes [ 16 ]. Extensions of IDO Core covering specific infectious diseases are created, first, by importing needed terms from IDO Core and other OBO Foundry ontologies, and second, by constructing the domain-specific terms where needed to adequately characterize entities in the relevant domain. Fig 1 illustrates example extensions, such as the Brucellosis Infectious Disease Ontology (IDOBRU; https://bioportal.bioontology.org/ontologies/IDOBRU ) the Influenza Infectious Disease Ontology (IDOFLU; https://bioportal.bioontology.org/ontologies/FLU ), and more recently the Coronavirus Infectious Disease Ontology (CIDO; https://bioportal.bioontology.org/ontologies/CIDO ). Each aims to be semantically interoperable with OBO library ontologies [ 11 , 34 – 36 ]. VIDO was designed to occupy the ontological space between such virus-specific ontologies and IDO Core. As a result, more specific virus-related ontologies such as CIDO [ 37 ] and IDOFLU are being curated to extend directly from VIDO, rather than directly from IDO Core.
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0285093.g001
The Virus Infectious Disease Ontology
VIDO takes IDO Core as its starting point, but also imports terms relevant to the domain of viruses from several other OBO Foundry ontologies, such as GO, the Ontology for General Medical Science (OGMS; https://bioportal.bioontology.org/ontologies/OGMS ) and the Ontology for Biomedical Investigation (OBI; https://bioportal.bioontology.org/ontologies/OBI ) [ 14 ]. The color- coded Fig 1 illustrates several importing relationships which provide the basis for VIDO definitions which we examine in what follows.
Acellular structure.
Like IDO Core, VIDO imports from OGMS. Examples of such imported terms are:
disorder = def Material entity that is a clinically abnormal part of an extended organism.
A part of a material entity is “clinically abnormal” if it is not expected in the life plan for entities of the relevant type and is causally linked to elevated risk–that is, risk exceeding some threshold–of illness, death, or disfunction [ 38 ]. Extended organism is imported from OGMS and organism from OBI, where they are defined as follows:
extended organism = def An object aggregate consisting of an organism and all material entities located within the organism, overlapping the organism, or occupying sites formed in part by the organism.
organism = def A material entity that is an individual living system, such as animal, plant, bacteria, or virus, that is capable of replicating or reproducing, growth and maintenance in the right environment. An organism may be unicellular or made up, like humans, of many billions of cells divided into specialized tissues and organs.
Here we run into the first of several ontological puzzles that emerged while developing VIDO. On the one hand, this definition aligns with common usage of the term “organism” among researchers for whom its instances are cellular entities [ 39 , 40 ]. On the other hand, the textual definition includes viruses among its instances, which are in every case acellular. Debates over organism ( https://github.com/OBOFoundry/COB/issues/6 ) among ontology developers have resulted in deprecation of the OBI term in favor of a nearby term from the Common Anatomy Reference Ontology ( https://bioportal.bioontology.org/ontologies/CARO ) with the label: organism or virus or viroid . At first glance, this appears to avoid the preceding worries, but further inspection reveals that this class is annotated as being an “exact synonym” of organism, and so suffers from the same issues raised above. Even if we put aside this latter issue, however, there are still two further concerns. First, introducing disjunctive classes is ad hoc [ 10 ]. Second, this disjunctive class leads naturally to debates over whether viruses are alive, since it classifies viruses alongside paradigmatic living entities. Decades of discussion have not resolved this question [ 41 – 46 ], and it is not obvious that we need an answer for the purposes of ontological modelling. It is unclear where consensus will land; in the interest of future-proofing our ontologies we should provide virus content in a way that neutral in regard to this issue to the maximal extent that this is possible. Rather than introduce an ad hoc disjunctive class, IDO Core, VIDO, and CIDO developers collaborated to add the following classes to IDO Core [ 47 ]:
self-replicating organic structure = def Object consisting of an organic structure that is able to initiate replication of its structure in a host.
acellular self-replicating organic structure = def Self-replicating organic structure comprised of acellular organic parts.
Which is imported to VIDO as the parent class of the term virus . The term virus is imported from the NCBITaxon [ 48 ] ( https://bioportal.bioontology.org/ontologies/NCBITAXON ), alongside terms relevant to virology, such as prion and satellite .
The NCBITaxon provides an extensive list of life science terms, but it has its limitations. As discussed in [ 16 ], NCBITaxon categorizes virus terms using the International Committee on Taxonomy of Viruses (ICTV). While an impressive taxonomy, the ICTV exhibits gaps in virus classification [ 49 , 50 ]. Additionally, NCBITaxon combined with standard ontology engineering tools such as Ontofox ( http://ontofox.hegroup.org/ ) [ 51 ] often leads to ontology developers importing superfluous portions of ICTV structured hierarchies, resulting in overwhelming taxonomies that are challenging for users to navigate. Fig 2 illustrates such a taxonomy found in IDOBRU, but importing an entire ICTV hierarchy is not uncommon (see for example the Schistosomiasis Ontology (IDOSCHISTO) [ 16 ]). Lastly, the NCBITaxon does not provide textual definitions for most terms within its scope. As stated, we seek to respect OBO Foundry metadata conventions [ 30 ] and ontology engineering best practices [ 10 , 31 ]; consequently, virus and other terminological content in VIDO must have textual definitions supplied.
https://doi.org/10.1371/journal.pone.0285093.g002
Standard definitions of “virus” provide a starting point for a textual definition, but caution is once again needed. Viruses are often described as obligate pathogens [ 52 , 53 ], since virus replication requires host machinery for production and assembly of viral components. However, defining a class virus solely in terms of what viruses typically do runs the risk of overlooking what viruses are, materially speaking. Compare: Homo sapiens are obligate aerobes, but this is no definition of the class. Insofar as we are defining the virus class, it is better to attend to genetic and structural components common to all viruses, and best to define the material entity in a way that captures obligate pathogenicity. VIDO accordingly defines:
virus = def Acellular self-replicating organic structure with RNA or DNA genetic material which uses host metabolic resources for RNA or DNA replication.
VIDO developers have contacted NCBITaxon developers proposing that the definitions we provide below be added to respective NCBITaxon terms. Other requests have been submitted, for example, to update the label of the NCBITaxon term from “Viruses” to “virus” in order to avoid ambiguous reference between classes and their instances [ 10 ].
Rather than import in accordance with the ICTV taxonomy, subclasses of virus are imported from NCBITaxon in alignment with the Baltimore Classification [ 54 ], which groups viruses into seven exhaustive classes based on genetic structure. For example, one subclass of virus is:
positive-sense single-stranded RNA virus = def Virus with genetic material encoded in single-stranded RNA that can be translated directly into proteins.
Which is a class representing one of the seven Baltimore Classification categories. These Baltimore Classification classes provide parent classes from which terms for more specific viruses can extend. Fig 3 illustrates how the Baltimore Classification appears in the Protégé visualization of the class positive-sense single-stranded RNA virus alongside viral replication pathways underwritten by genetic differences in viruses.
https://doi.org/10.1371/journal.pone.0285093.g003
By incorporating the Baltimore Classification, we provide developers of virus-specific domain ontologies an ontological representation of viruses that is simpler and easier to navigate than the ICTV, which currently underwrites the NCBITaxon.
VIDO subclasses of virus include those common in virology research, such as bacteriophage– viruses that infect bacteria– virophage– viruses that infect viruses– oncovirus– viruses that cause cancer–and mycovirus– viruses that infect fungi. As well as:
virion = def Virus that is in its assembled state consisting of genomic material (DNA or RNA) surrounded by coating molecules.
Some researchers use “virion” and “virus” synonymously [ 55 ]. Some define “virion” so that instances only exist outside host cells [ 56 ], or they distinguish virions outside host cells from those inside host cells, calling the former “mature virions.” Some claim “virion” is best understood as analogous to a sperm cell [ 57 , 58 ]. Ontologically speaking, one might model the relationship between a virus and its virion in a variety of ways: virion is to a virus as human infant is to human, or as human student is to human, or as human gamete is to human. Treating virions as akin to gametes is uncommon among researchers. Between the remaining options, we adopt the first, treating virion as a type of virus , since adopting the alternative would suggest a virion is simply a virus that is in a specific context, a result that overlooks the importance of genomic assembly to identifying virions.
Incidentally, some viruses do not replicate faithfully, perhaps resulting in genetically distinct mutants or–in extreme cases–an inactive aggregate of virion components. Virus mutations may undermine host immune system recognition of viral threats, as evidenced by the difficulty in developing vaccines for certain influenza strains. If there are too many mutations, however, then a virus may lose its ability to replicate, an observation used in development of treatments for polio and hepatitis C which exacerbate respective virus mutations [ 59 , 60 ]. VIDO thus provides the term:
disordered virus = def Acellular self-replicating organic structure having some clinically abnormal arrangement of viral components (e.g. viral capsid, viral DNA/RNA).
Viruses falling in this class may be associated with diseases much different from those associated with viruses that do not exhibit disorder. Terms for viral components are imported to VIDO: from GO, viral nucleocapsid , viral capsid , capsomere , viral envelope ; from the Chemical Entities of Biological Interest (ChEBI) ontology ( https://bioportal.bioontology.org/ontologies/CHEBI ) [ 61 , 62 ], nucleic acid and ribonucleic acid ; from the Protein Ontology ( https://bioportal.bioontology.org/ontologies/PR ), protein and viral protein .
Infectious structure.
The term “pathogen” is indexed to species or to stages in the developmental cycle of a species. A given virus may engage in mutual symbiosis with one species, while exhibiting pathogenic behavior towards others [ 63 , 64 ]. Mature plants are often susceptible to different pathogens than developing plants [ 65 – 68 ]. We capture virus pathogenicity in VIDO in steps. From IDO Core [ 16 ], we import dispositions borne by pathogens and infectious agents, as follows:
pathogenic disposition = def Disposition borne by a material entity to establish localization in, or produce toxins that can be transmitted to, an organism or acellular structure, either of which may form disorder in the entity or immunocompetent members of the entity’s species.
infectious disposition = def Pathogenic disposition borne by a pathogen to be transmitted to a host and then become part of an infection in that host or in immunocompetent members of the same species as the host.
The class infectious agent in IDO Core is a subclass of organism , and so cannot include instances of virus . To address this issue, the term infectious structure was developed to parallel the IDO Core term infectious agent and to provide a logically defined subclass of acellular self-replicating organic structure . The term infectious disposition bridges infectious acellular structures and infectious organisms since instances of each bear an infectious disposition. Moreover, the logical definitions of infectious structure and infectious agent are such that, though the former is a defined subclass of acellular self-replicating organic structure and the latter a subclass of organism , they are both inferred subclasses of pathogen .
As discussed in [ 16 ], establishment of localization used in pathogenic disposition is characterized using the IDO Core term establishment of localization in host representing tethering or adhesion to a host, while “formation of disorder” abbreviates appearance of disorder , which is a process that results in formation of a disorder . The definition of pathogenic disposition is meant to reflect a temporal ordering between establishment of localization and appearance of disorder. This is reflected explicitly in the logical axioms associated with the class. Similarly, in the definition of infectious disposition there is an intended temporal ordering between transmission to a host–represented by pathogen transmission process imported from the Pathogen Transmission Ontology ( https://bioportal.bioontology.org/ontologies/PTRANS )–and becoming part of an infection–represented by the IDO Core process of establishing an infection . A pathogen bearing an infectious disposition that generates disorder in a host will have been transmitted to the host prior to establishing localization in the host and will have established an infection prior to the appearance of disorder.
The complexity of the definitions of pathogenic disposition and infectious disposition reflect the variety of pathogen examples documented in contemporary literature. Consider S . aureus , an opportunistic pathogen [ 56 ] in humans. We count S . aureus as a pathogen, even when it does not realize disorder in a host, since it is nevertheless disposed to localize in a human host and generate disorder if given the opportunity. This is a BFO disposition of S . aureus as it is an “internally-grounded” property of the entity [ 32 ]. That is, it is part of the material basis of S . aureus to generate disorder in human hosts if given the chance. This is analogous to the way salt has a disposition to dissolve, based on its lattice structure, independently of whether it ever realizes this disposition. Opportunistic pathogens are not pathogens because of an opportunity; they are pathogens because they are disposed to localize and cause disorder in a host.
Consider now, C . botulinum , a pathogen which produces a toxin and which may produce a spore ingested by humans. This bacterium is a pathogen for adult humans since the toxins often result in disorder when ingested. Furthermore, C . botulinum may cause infection in human infants if, say honey colonized by C . botulinum is ingested. The sugar content of honey inhibits C . botulinum growth, but in the low-oxygen, low-acid intestines of human infants, spores can localize, grow, and produce toxins resulting in disorder. Thus, C . botulinum counts as a human infant pathogen. Nevertheless, because C . botulinum is not itself disposed to invade or be transmitted to human infants, we do not say the bacterium is infectious [ 69 ]. Being part of an infection is not itself sufficient for something to be counted as infectious. Pathogens bearing an infectious disposition must be disposed to both transmit and become part of an infection. Many opportunistic pathogens, for example, are not infectious.
Consider lastly, the respective definitions of infectious disposition and pathogenic disposition address instances where mutations in hosts may block realization of disorder or infection. In such cases, an infectious pathogen may nevertheless be transmissible and cause disorder or infection in others. For example, HIV-1 is a pathogen that may localize in a host with CCR-5 mutations [ 70 ] that block the virus from attaching to host cells, and so block pathogenesis to AIDS. Similarly, P . falciparum may be transmitted to a host with a sickle-cell trait that blocks manifestation of the disease malaria [ 71 , 72 ]. However, P . falciparum and HIV-1 count as pathogens even if they do not result in the formation of disorders for hosts with a sickle-cell trait or CCR-5 mutation, respectively. IDO Core reflects this characterization. Each pathogen may be transmitted to immunocompetent members of the same species as the host, and so count as bearing instances of infectious disposition and pathogenic disposition . Note, the fact that P . falciparum and HIV-1 do not result in the formation of disorders in hosts with sickle-cell traits or CCR-5 mutations should not suggest that there are no clinical abnormalities associated with these traits or mutations. Individuals with, say, CCR-5 mutations do exhibit clinical abnormalities, and so do exhibit disorders. But these disorders are due to the CCR-5 mutation rather than the HIV-1 infection.
Whenever an infectious disposition is realized, this is always in some site in a host, via some transmission to that host, and some generation of infection and disorder in that host. Infectious structures– such as viruses–bear this disposition. For example, each SARS-CoV-2 virus is disposed to be transmitted to hosts, localize, cause infection, and result in disorder.
Pathogen host.
Until recently, microbiologists, immunologists, virologists, and others studying pathogenesis have engaged in either host-centered or pathogen-centered pathogenesis research [ 73 – 77 ]. Each approach has led to impressive research results. But emphasizing one aspect of host-pathogen interactions at the expense of the other may leave valuable questions unanswered [ 78 ]. Emphasis, for example, solely on pathogenic factors of SARS-CoV-2 will provide only a partial explanation of various pathogenesis pathways observed in clinical settings. IDO Core and VIDO prioritize neither host nor pathogen in representation of pathogens and associated diseases, adopting the Damage Response Framework (DRF) for guidance in development of relevant terms [ 79 – 82 ]. According to the DRF, pathogenesis results from interactions between both host and pathogen interacting primarily through host damage, which is a function of the intensity and degree of host response and pathogen factors. Host and pathogen interactions thus influence manifestations of signs, symptoms, and disease. IDO Core now defines hosts and pathogens in terms of roles and allows that acellular structures may also be pathogen hosts, such as when a virus hosts a virophage:
host role = def Role borne by either an organism whose extended organism contains a distinct material entity, or an acellular structure containing a distinct material entity, realized in use of that structure or organism as a site of reproduction or replication.
pathogen host role = def Host role borne by an organism or acellular structure having a pathogen as part.
Following BFO, roles are “externally grounded” realizable entities that may be gained or lost based on circumstance without necessarily involving material change to their bearer, such as the role a student acquired once enrolled in a university.
The Symptom Ontology ( https://bioportal.bioontology.org/ontologies/SYMP ) provides a extensive terminological content for representing symptoms owing to viral infection, such as fever , taste alteration , and so on [ 83 ]. Given the importance of asymptomatic carriers in viral infection spread, moreover, attention is also given in IDO Core to:
symptomatic carrier role = def Pathogen host role borne by an organism whose extended organism contains a pathogen bearing an infectious disposition towards the host, and the host has manifested symptoms of the infectious disease caused by the pathogen.
asymptomatic carrier role = def Pathogen host role borne by an organism whose extended organism contains a pathogen bearing an infectious disposition towards the host, and the host has no symptoms of the infectious disease caused by the pathogen.
subclinical infection = def Infection that is part of an asymptomatic carrier.
The definition of the term subclinical infection reflects standard use of the terms “subclinical” and “asymptomatic” while allowing for asymptomatic clinically abnormal infections. VIDO extends subclinical infection to subclinical virus infection , namely, those subclinical infections caused by a virus. These remarks bring us full circle to the term disorder introduced above, since clinical abnormality is associated with disorder. When that disorder stems from infection it counts as an:
infectious disorder = def Disorder that is part of an extended organism which has an infectious pathogen part, that exists as a result of a process of formation of disorder initiated by the infectious pathogen.
And when the adverted pathogen is a virus, it falls in the VIDO class:
virus disorder = def Infectious disorder that exists as a result of a process of formation of disorder initiated by a virus.
Viral disease.
Medical researchers draw a distinction between symptoms and signs, a distinction which OBO Foundry ontologies respect (from OGMS [ 38 ]):
symptom = def Process experienced by the patient which can only be experienced by the patient, that is hypothesized to be clinically relevant.
qualitative sign = def Abnormal observable quality of a part of a patient that is hypothesized to be clinically relevant.
processual sign = def Abnormal processual entity occurring in a patient that is hypothesized to be clinically relevant.
An asymptomatic carrier infected with SARS-CoV-2 likely exhibits signs indicating that the infection is clinically abnormal, such as ground-glass opacities. Such asymptomatic carriers exhibit an instance of the VIDO class virus disorder which is the material basis of a viral disease :
infectious disease = def Disease whose physical basis is an infectious disorder.
viral disease = def Infectious disease inhering in a virus disorder that is a disorder due to the presence of the virus.
Worth noting is that these definitions are consistent with the CDC’s case criteria definitions adopted between April 5, 2020 and February 28, 2023, which indicate that the presence of the SARS-CoV-2 genome or relevant antigens in an individual is sufficient to count as a case of COVID-19, asymptomatic or not [ 84 – 86 ]. A viral disease may be realized in a disease course:
infectious disease course = def Disease course that is the realization of an infectious disease.
viral disease course = def Infectious disease course whose physical basis is a virus disorder that is clinically abnormal in virtue of the presence of the relevant virus.
Here infectious disease and infectious disease course are imported from IDO Core, and are subclasses, respectively, of disease and disease course , which are imported from OGMS.
Viral epidemiology.
Changes in viral disease and infection incidence are among the targets of epidemiological investigation. VIDO imports from IDO Core:
infectious disease incidence = def Quality that inheres in an organism population and is the number of realizations of an infectious disease for which the infectious disease course begins during a specified period.
infectious disease incidence rate = def Quality that inheres in an organism population and is the infectious disease incidence proportion per unit time.
infectious disease incidence proportion = def Quality that inheres in an organism population and is the proportion of members of the population not experiencing an infectious disease course at the beginning of a specified period and in whom the infectious disease begins during the specified period.
organism population = def Aggregate of organisms of the same species.
Additionally, VIDO imports from IDO Core other important epidemiological terms, such as infection prevalence , infectivity , and infectious disease mortality rate . Each are specifically dependent entities inhering in some material entity, though not always in some organism population. For example, infectivity is a quality inhering in instances of pathogen . Additionally, VIDO imports from IDO Core:
infection incidence = def Quality that inheres in an organism population and is the number of organisms in the population that become infected with a pathogen during a specified period.
on which infectious disease incidences depend, as infectious disease realizations require infection.
A quality process profile is a type of process which tracks changes of specific qualities in material entities over time [ 26 ]. For example, a patient’s temperature will likely fluctuate over time, as will many other qualities of the patient. The specific fluctuations of temperature in the patient over time is a process profile , which reflects common abstractions used in clinical diagnosis and testing and manifesting in charts prepared from time-series data. Changes in qualities of clinical interest may follow several patterns, each of which can be defined as a subclass of process profile . A patient’s temperature may exhibit a linear increase followed by a linear decrease. Similarly, there are process profile instances of cyclical patterns, for instance the seasonal patterns of influenza [ 87 ]. Such patterns can be tracked in VIDO by classes such as:
viral disease incidence profile = def Infectious disease incidence profile comprised of a series of determinate viral disease incidence qualities caused by a specific virus in a population over time.
viral disease incidence proportion profile = def Infectious disease incidence proportion profile comprised of a series of viral disease incidence proportion qualities caused by a specific virus per unit time.
viral disease incidence rate profile = def Infectious disease incidence rate profile comprised of a series of viral disease rate qualities caused by a specific virus per unit time.
Extending VIDO to CIDO
VIDO serves as a bridge between IDO Core and the IDO extension ontologies representing specific viral diseases. Of particular importance during the pandemic has been the Coronavirus Infectious Disease Ontology (CIDO; https://bioportal.bioontology.org/ontologies/CIDO ), developed by the He Group at the University of Michigan. CIDO provides terminological content that facilitates representations of coronavirus genome, protein structures, epidemiological surveillance, vaccine development, and treatment options. The ontology has been used to annotate data pertaining to 136 known anti-coronavirus drugs [ 6 ], as well as in the identification of approximately 110 candidate drugs [ 7 ] for potential drug repurposing projects with respect to COVID-19 [ 88 ]. More recently, CIDO has been employed as a general framework for understanding host-pathogen interactions [ 78 ]. IDO Core, VIDO, and CIDO development teams work together closely in the interest of ensuring ontology alignment.
CIDO will extend from VIDO by adopting, among other terms:
coronavirus disease = def Viral disease inhering in a coronavirus disorder.
coronavirus disease course = def Viral disease course that is the realization of some coronavirus disease and has as a participant a coronavirus.
This extension example illustrates a downward population recipe useful for aligning CIDO terms to VIDO, by starting with a given virus term from the latter, and restricting subclasses based on features of coronaviruses and associated diseases. Moreover, common coronavirus features can be reused from OBO ontologies to complement the CIDO characterization of the virus, such as the viral envelop glycoprotein spikes [ 89 , 90 ]. Some such terms are specializations of terms from the Protein Ontology ( https://bioportal.bioontology.org/ontologies/PRO ), e.g. spike glycoprotein (SARS- CoV-2) . CIDO covers existing and novel coronaviruses in general, and so provides resources for detailed comparison of coronavirus biological profiles. Fig 4 illustrates various links between VIDO and CIDO, and the IDO Core and GO ontologies.
https://doi.org/10.1371/journal.pone.0285093.g004
SARS-CoV-2 pathogenesis.
Characterizing pathogenesis to COVID-19 is aided by terms such as:
COVID-19 = def Coronavirus disease inhering in a SARS-CoV-2 disorder.
COVID-19 disease course = def Coronavirus disease course that is the realization of some COVID-19 disease and has participant SARS-CoV-2.
Ontologically precise representation of COVID-19 pathogenesis is crucial for understanding the range of symptoms and signs which appear across demographics [ 91 – 94 ]. Ontological representation of COVID-19 pathogenesis is aided by reusing OBO Foundry ontology terms, resulting in the following definition:
SARS-CoV-2 pathogenesis = def Coronavirus pathogenesis process realization of an infectious
disposition inhering in SARS-CoV-2 or a SARS-CoV-2 population, having at least the proper process parts:
- pathogen transmission,
- establishment of localization in host,
- process of establishing an infection, and
- appearance of a virus disorder.
Instances of SARS-CoV-2 pathogenesis are asserted as part of some COVID-19 disease course . The term coronavirus pathogenesis will ultimately be imported to CIDO, as a subclass of the VIDO term viral pathogenesis , itself a subclass of:
pathogenesis = def Process that generates the ability of a pathogen to induce disorder in an organism.
which is imported from GO. As defined, pathogenesis is a success term [ 25 ], in that it encompasses formation of disorder in an entity. Of course, this is not meant to imply that SARS-CoV-2 infections necessarily lead to successful pathogenesis. SARS-CoV-2 infections, for example, may not lead to host disorder, in which case there would be no pathogenesis. Just as important as it is to represent SARS-CoV-2 pathogenesis to COVID-19, adequate representation of the target domain requires representation of pathogenesis to acute respiratory distress syndrome (ARDS), one of the leading causes of death in those infected by SARS-CoV-2 [ 95 , 96 ]:
acute respiratory distress syndrome = def Progressive and life-threatening pulmonary distress in the absence of an underlying pulmonary condition, usually following major trauma or surgery.
which may be imported from the Experimental Factor Ontology ( https://bioportal.bioontology.org/ontologies/EFO ). Similar remarks apply to other diseases associated with SARS-CoV-2 pathogenesis.
SARS-CoV-2 pathogenesis involves transmission of SARS-CoV-2 virions. From PTRANS ( https://bioportal.bioontology.org/ontologies/PTRANS ) is imported:
pathogen transmission process = def Process during which a pathogen is transmitted directly or indirectly to a new host.
From which SARS-CoV-2 specific terms can be constructed. Additionally, IDO Core provides important role terms relevant to pathogen transmission, such as:
pathogen transporter role = def Role borne by a material entity in or on which a pathogen is located, from which the pathogen may be transmitted to a new host.
An important subclass fomite role– roughly, a pathogen transporter role borne by a non-living entity–may feature in SARS-CoV-2 transmission via instances of fomite role bearing:
respiratory droplet = def Respiratory secretion composed of a bounded portion of liquid which maintains its shape due to surface tension.
respiratory droplet SARS-CoV-2 fomite = def Respiratory droplet fomite with SARS-CoV-2 part.
Knowledge of transmission steps supports strategies designed to break the transmission chain.
Worth noting is that the OBO library ontology APOLLO-SV ( https://bioportal.bioontology.org/ontologies/APOLLO-SV ) also contains terms, such as contact tracing and quarantine control strategy , which may be leveraged to represent virus-specific transmission control strategies.
SARS-CoV-2 replication.
SARS-CoV-2 pathogenesis involves replication in a host. The term virus replication is defined in VIDO as a subclass of the IDO Core term replication , specifically:
virus replication = def Replication process in which a virus containing some portion of genetic material inherited from a parent virus is replicated.
And instances of viral disease course and virus pathogenesis have virus replication as parts. SARS-CoV-2 replication occurs within an:
incubation process = def Process beginning with the establishment of an infection in a host and ending with the onset of symptoms by the host, during which pathogens are multiplying in the host.
Which occupies an incubation interval and may precede a communicability interval . The corresponding process during which SARS-CoV-2 hosts bear a contagiousness disposition has proper part some latency process which itself has an eclipse process as part::
communicability interval = def One-dimensional temporal region during which a pathogen host bears a contagiousness disposition.
latency process = def Process beginning with the establishing of an infection in a host and ending when the host becomes contagious, during which pathogens are multiplying in the host.
eclipse process = def Process beginning with the establishment of a virus in a host and ending with the first appearance of a virion following viral release, during which an infecting virus is uncoating to begin genome replication.
The last are specific to viruses, and so specific to VIDO. Viral dormancy is a virus-specific term from VIDO occurring over a:
viral dormancy interval = def One-dimensional temporal region on which a virus is no longer replicating but remains within a host cell and which may be reactivated to begin replication again.
Viral dormancy is characteristic of familiar viruses such as varicella zoster and herpes simplex .
VIDO includes as a temporal subdivision of a virus developmental process:
virus generative stage = def Infectious structure generative stage that is a temporal subdivision of a virus developmental process.
Subclasses of which include the stages through which viruses may proceed during replication:
virus attachment stage = def Virus generative stage during which a virion protein binds to molecules on the host surface or host cell surface projection.
virus penetration stage = def Virus generative stage during which a virion or viral nucleic acid breaches the barriers of a host.
SARS-CoV-2 attachment stage = def Virus attachment stage during which SARS-CoV-2 bonds with a host cell.
SARS-CoV-2 penetration stage = def Virus penetration stage during which SARS-CoV-2 penetrates a host cell.
SARS-CoV-2 susceptibility.
Only cells with certain features are susceptible to SARS-CoV-2 infection [ 16 ]. For example, successful infection in humans typically involves SARS-CoV-2 attachment to alveolar epithelial cells through angiotensin-converting enzyme 2 (ACE2) receptors [ 97 – 99 ]. Cells lacking ACE2 receptors seem protected from attachment by SARS-CoV-2. Those with receptors can be represented in CIDO using:
SARS-CoV-2 adhesion susceptible cell = def Virus adhesion susceptible cell with a functional receptor part bearing an adhesion disposition realized in a SARS-CoV-2 attachment stage.
adhesion disposition = def Disposition borne by a macromolecule that is the disposition to participate in an adhesion process.
Where adhesion disposition is imported from IDO Core and virus adhesion susceptible cell defined in VIDO. The ACE2 functional receptor is defined in the Protein Ontology ( https://bioportal.bioontology.org/ontologies/PRO ):
angiotensin-converting enzyme 2 = def A protein that is a translation product of the human ACE2 gene or a 1:1 ortholog thereof.
Attachment is frequently followed by cell penetration, where cell cleavage is aided by transmembrane protease serine 2 (TMPRSS2) prior to SARS-CoV-2 cell membrane fusion [ 100 , 101 ]. These observations motivate introducing terminological content for defining SARS-CoV-2 penetration susceptible cell such as:
SARS-CoV-2 penetration disposition = def Virus penetration disposition borne by a functional receptor complex that is the disposition to participate in a SARS-CoV-2 penetration process.
Ontological representation of the SARS-CoV-2 replication cycle provides targets for disruption or regulation of that cycle, which is important to rational drug design [ 102 – 106 ]:
negative regulation of SARS-CoV-2 attachment = def Negative regulation of coronavirus replication process that stops, prevents, or reduces the frequency of some SARS-CoV- 2 attachment stage.
negative regulation of SARS-CoV-2 penetration = def Negative regulation of coronavirus replication that stops, prevents, or reduces the frequency of some SARS-CoV-2 penetration stage.
Following our strategy of linking VIDO and CIDO, parent classes of negative regulation of coronavirus classes have a proper home in CIDO, while their parent classes–negative regulation of viruses more generally–have a proper home in VIDO.
Annotations.
Coverage in VIDO and CIDO can be illustrated by annotation of coronavirus research articles.
Consider the following overview of SARS-CoV-2 pathogenesis (compare bold with Fig 5 ): Following replication , cell lysis of SARS-CoV-2 coronavirus virions causes host cells to release molecules which function to warn nearby cells . When recognized by epithelial cells , endothelial , and alveolar macrophages , proteins such as IL-6 , IP-10 , and MCPI , are released which attract T cells , macrophages , and monocytes to the site of infection , promoting inflammation . In disordered immune systems , immune cells accumulate in the lungs , then propagate to and damage other organs . In normal immune systems , inflammation attracts T cells which neutralize the virus at the site of infection . Antibodies circulate , preventing SARS-CoV-2 infection , and alveolar macrophages recognize SARS-CoV-2 and eliminate virions via phagocytosis [ 92 , 107 , 108 ].
https://doi.org/10.1371/journal.pone.0285093.g005
In a more ontologically oriented language, we speak of the relevant part of a host’s immune response as being disposed to manifest a response that eliminates SARS-CoV-2 infection, while SARS-CoV-2 has a disposition to block manifestation of this immune system response. Consider next a color-coded selection from the Lancet [ 109 ] concerning SARS-CoV-2:
“The viral load s in throat swab s and sputum sample s peaked at around 5–6 days after symptom onset , ranging from around 10^4 to 10^7 copies per mL during this time .”
SARS-CoV-2 infected hosts contain the highest concentration of SARS-CoV-2 virions–the viral load– during the incubation interval [ 110 ]. Viral load is a common measurement of the proportion of virions to fluid, and for SARS-CoV-2 is frequently measured from host sputum. VIDO provides the resources for annotating virus quantification:
viral load = def Quality inhering in a portion of fluid that is the proportion of virions to volume of that portion of fluid
Our color-coding of the above passage from the Lancet models term reuse across existing ontologies. For example, developers can use VIDO and CIDO terms alongside terms from the Common Core Ontology ( https://github/com/CommonCoreOntology/CommonCoreOntologies ) such as is measured by , measurement information content entity , has integer value , uses measurement unit , and milliliter measurement unit . Other virus quantification metric terms, such as multiplicity of viral infection ‐ the ratio of virions to susceptible cells in a target area–can be found in VIDO as well.
Motivated to standardize virus ontology extensions of IDO Core, we have developed VIDO and provided a recipe for connecting VIDO to more virus-specific extensions of IDO Core, illustrated by connecting VIDO to CIDO. Summarizing our results, we have introduced acellular structure as a parent class to virus , motivated using the Baltimore classification to model viruses rather than the International Committee on Taxonomy of Viruses classification, revised IDO Core’s pathogen and host classes to accommodate acellular structures, and extended IDO Core’s infectious disease , infectious disease course , and infectious disease epidemiology classes to cover viruses. We then introduced bridge classes in VIDO to better align IDO Core and CIDO, illustrating throughout how CIDO terminological content can be extended and enriched to represent SARS-CoV-2 pathogenesis, associated transmission processes, virus transporters, replication stages and associated temporal extents, as well as pathogenesis regulation. Our attention was then turned to annotations of texts concerning SARS-CoV-2 and COVID-19, by which we highlighted how ontologies using common vocabularies may be seamlessly interwoven to provide broad annotation coverage for the domain.
VIDO and CIDO are not the only ontologies developed to support curation of COVID-19 data [ 111 – 114 ]. However, most alternatives are stand-alone initiatives, and so subject to the silo problems typically found in ontologies developed outside the scope of the OBO Foundry and with no attention to its principles. That said, VIDO and CIDO developers have participated in harmonization efforts aimed at semantic integration across COVID-19 ontologies [ 115 ]. Notably, harmonization efforts have resulted in the deprecation of the COVID-19 Infectious Disease Ontology (IDO-COVID-19)–introduced in a preprint version of the current paper [ 116 ]–with parties agreeing its scope was subsumed by CIDO. Additionally, VIDO and CIDO have been used to highlight ontology conflict resolution strategies [ 117 ]. It is not uncommon for ontology researchers working independently in nearby domains to construct overlapping ontology content.
The harmonization efforts of the VIDO and CIDO development teams signal to the wider ontology community our willingness to reuse terms where possible and obsolete terms or cede terms to other ontologies when needed.
VIDO and CIDO enable extensive representation of virus-related research. The very scope of VIDO provides challenges, however, as does the specificity of CIDO. For these reasons, attempts have been made to foster community-driven development of both. The development team for each ontology spanned disciplines in the life sciences, and to ensure the computational viability of the formal representation of each ontology, included specialists in logic. Often, terms were developed then presented to domain specialists for vetting, after which they were refined through discussion.
As in the case of all scientific ontologies, refinement will continue as research advances, and further collaborators are welcome. Interested parties may contact the corresponding author to be invited to on-going VIDO development meetings and may contact co-author He for invitation to development meetings concerning CIDO. Additionally, collaborators are encouraged to raise issues on respective GitHub issue trackers for VIDO ( https://github.com/infectious-disease-ontology-extensions/VIDO ) and CIDO ( https://github.com/CIDO-ontology/cido ).
The existence of IDO Core extensions covering infectious disease-causing entities other than viruses suggests a need for the creation of reference ontology extensions of IDO covering bacteria, fungi, and parasites. To that end, development of the Bacteria Infectious Disease Ontology is underway ( https://github.com/infectious-disease-ontology-extensions/Bacteria-Infectious-Disease- O ntology) as is the development of the Fungal Infectious Disease Ontology ( https://github.com/SydCo99/MIDO ). The methodology illustrated in the development of VIDO provides a recipe for such reference ontology creation. Additionally, the methodology illustrated in the development of CIDO provides a recipe for the creation of novel virus-specific ontologies, namely, by extending them from existing virus ontologies. Adoption of these methodologies by developers during ontology construction will significantly reduce the labor involved in ontology creation. Related, linking research on infectious diseases to developments on non-infectious diseases is no less important than our focus here. In this respect, VIDO and CIDO benefit from alignment with IDO Core, which itself aligns with the Ontology of General Medical Science (OGMS), whose scope extends beyond infectious disease. What this means in practice is that, for example, kidney disease [ 118 ] and cancer [ 119 ] researchers accurately using the OGMS vocabulary to represent data, invariably use ontology terms and methodologies common to IDO Core, VIDO, and CIDO, thereby lowering barriers to data integration and interoperability.
VIDO and CIDO are being used to annotate host-coronavirus protein-protein interactions, in the interest of developing more effective treatment strategies for those infected by SARS-CoV-2 or variants [ 37 ]. While various treatments have been authorized for emergency use [ 120 ], there is significant room for improvement. Rather than focus on a single drug to treat infected patients, VIDO and CIDO developers have pursued investigating drug cocktail strategies to improve treatment outcomes. Foundational to these investigations has been proper characterization of viral proteins playing different roles in host-coronavirus interactions which impact pathogenesis [ 78 ].
From another direction, VIDO and CIDO have been used in automated electronic health-record annotation [ 121 ], in particular those involving COVID-19 data, which highlights the importance of providing researchers with terminological content relevant to nearby domains. Recent developments at the intersection of ontology engineering and machine learning research have, moreover, motivated the need for formally well-defined ontologies in machine learning pipelines using minimal data [ 122 ]. By exploiting formal axioms in, for example, the Gene Ontology, impressive zero-shot predictions for protein functions can be generated [ 123 ]. We believe the formal axiomatization of VIDO makes it a particularly promising ontology for inclusion in zero-shot research and intend to explore how VIDO may supplement machine learning efforts in future work.
Ontologies provide important tools for overcoming contemporary big data challenges. It is incumbent on working ontologists representing life science research to seek harmonization with nearby ontologies, else we run the risk of reinstating the same big data challenges ontologies have previously been so successful at addressing. VIDO represents a substantial effort to characterize viruses in general, in a collaborative, computationally tractable manner. CIDO too represents a significant effort to characterize coronaviruses in a specific, no less collaborative, no less computationally tractable manner. Connecting VIDO to CIDO improves semantic interoperability among IDO Core-conformant infectious disease ontologies and, moreover, improves interoperability with other BFO-conformant ontologies, ranging from the OBO Foundry to numerous other ontology projects employing BFO as a top-level architecture. Consequently, our work provides researchers resources for gathering and coordinating life science data while avoiding issues that so frequently undermine automating integration and analyses of the data flood in which we so often find ourselves [ 124 – 126 ].
Acknowledgments
Many thanks to Asiyah Yu Lin for assistance in VIDO development and harmonization; to Darren Natale and Sydney Cohen for helpful critical feedback on earlier drafts; to Amanda Hicks and Neil Otte for comments on the VIDO rdf files; to participants at the 2022 International Conference on Biomedical Ontologies, with particular thanks to Alexander Diehl and Chris Stoeckert for their helpful feedback before, during, and after the conference. Figures were designed by or in consultation with Rain Yuan.
- View Article
- PubMed/NCBI
- Google Scholar
- 10. Arp R, Smith B, Spear A. (2015) Building Ontologies with Basic Formal Ontology. Cambridge, MA: MIT Press.
- 15. Cowell LG, Smith B. (2010) Infectious Diseases Ontology. In: Sintchenko V, editor. Infectious Disease Informatics. New York, NY: Springer. p. 373–95.
- 18. The Open Biomedical Ontologies Foundry. http://obofoundry.org/ Accessed 11 Mar 2023.
- 20. Baader F, Horrocks I, Lutz C, Sattler U. (2017). An Introduction to Description Logic. Cambridge University Press. https://doi.org/10.1017/9781139025355
- 21. Homer H, Selman AL. (2011) Computability and Complexity Theory: Texts in Computer Science. 2nd Edition. Springer.
- 27. Smith B. (2012) On Classifying Material Entities in Basic Formal Ontology. Interdisciplinary Ontology: Proceedings of the Third Interdisciplinary Ontology Meeting. Tokyo: Keio University Press. 1–13.
- 32. Goldfain A, Smith B, Cowell LG. (2010) Dispositions and the infectious disease ontology. In: Galton A, Mizoguchi R, editors. Formal Ontology in Information Systems: Proceedings of the 6th International Conference (FOIS 2010). Amsterdam: IOS Press.
- 44. Claverie JM. (2008) Encyclopedia of Virology 3rd Edition.
- 47. He Y. (2022). Ontological Classification of Self-replicating Organic Structures. Preprint. 1–8. https://doi.org/10.31219/osf.io/n2zkh
- 52. Dimmock NJ, et. al. (2007). Introduction to Modern Virology. 6th edition. Blackwell Publishing.
- 56. Bauman R. (2017). Microbiology with Disease Taxonomy. Pearson Publishing.
- 69. Ananthanarayan R., Paniker J. (2005). Textbook of Microbiology. 7th Edition.
- 78. Yu H, et al. (2022) A New Framework for Host-Pathogen Interaction Research. Frontiers in Immunology. https://doi.org/10.3389/fimmu.2022.1066733
- 84. Centers for Disease Control and Prevention. (2020). Coronavirus Disease 2019 (COVID-19) 2020 Interim Case Definition, Approved April 2, 2020. https://ndc.services.cdc.gov/case-definitions/coronavirus-disease-2019-2020/ Accessed Mar 11 2023.
- 85. Council of State of Territorial Epidemiologists. (2023). Standardization Surveillance Case Definition and National Notification for 2019 Coronavirus Disease (COVID-19). https://ndc.services.cdc.gov/conditions/coronavirus-disease-2019-covid-19/ Accessed Mar 11 2023.
- 86. Centers for Disease Control and Prevention. (2023). Coronavirus Disease 2019 (COVID-19) 2023 Case Definition, Approved February 28, 2023. https://ndc.services.cdc.gov/case-definitions/coronavirus-disease-2019-covid-19/ Accessed Mar 11 2023.
- 103. Moderna Announces Phase 3 COVE Study of mRNA Vaccine against COVID-19 (mRNA-1273) Begins. Press Release (2020); https://investors.modernatx.com/news-releases/news-release-details/moderna-announces-phase-3-cove-study-mrna-vaccine-against-covid.
- 104. A Study to Evaluate Efficacy, Safety, and Immunogenicity of mRNA-1273 Vaccine in Adults Aged 18 Years and Older to Prevent COVID-19, ClinicalTrials.gov Identifier: NCT04470427, https://clinicaltrials.gov/ct2/show/NCT04470427 .
- 105. Pfizer and Biotech Choose Lead mRNA Vaccine Candidate against COVID-19 and Commence Pivotal Phase 2/3 Global Study. Press Release. (2020). https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-choose-lead-mrna-vaccine-candidate-0
- 111. WHO COVID-19 Rapid Version CRF. https://bioportal.bioontology.org/ontologies/COVIDCRFRAPID Accessed Mar 11 2023.
- 112. COVID-19 Surveillance Ontology. https://bioportal.bioontology.org/ontologies/COVID19 Accessed Mar 11 2023.
- 113. Linked COVID-19 Data Ontology. https://github.com/Research-Squirrel-Engineers/COVID-19 . Accessed Mar 11 2023.
- 114. COVID-19 Research Knowledge Graph. https://github.com/nasa-jpl-cord-19/covid19-knowledge-graph . Accessed Mar 11 2023.
- 124. Apolinario-Arzube Ó. et al. (2020) CollaborativeHealth: Smart Technologies to Surveil Outbreaks of Infectious Diseases Through Direct and Indirect Citizen Participation. In: Silhavy R. (eds) Applied Informatics and Cybernetics in Intelligent Systems. Advances in Intelligent Systems and Computing. 1226. https://doi.org/10.1007/978-3-030-51974-2_1

- Faculty Directory
- Virology Training Grant
- Graduate Programs
- Undergraduate Program
- Facilities & Services
Welcome to the Center for Virus Research
Current topics in virus research.

A new threat?

Virus Research News
Congratulations to rémi buisson and sunwoo oh new antiviral therapies on horizon thanks to medical discovery, uci cfccc beyond cancer speaker series, cncm is awarded $1.4 million nih grant for alzheimer’s disease research training, congratulations to travis wiles, uc irvine-led research team creates novel rabies viral vectors for neural circuit mapping, congratulations to nir drayman.
The Center for Virus Research (CVR) aims to foster scholarship, training, and collaborative research at UCI across many disciplines, based on studies founded in molecular virology.
Center Administration

Thomas E. Lane, Ph.D. Center Director [email protected]

Matthew D. Marsden, Ph.D. Center Associate Director [email protected]

Rebecca Taylor Center Administrator [email protected]
Executive Committee

Matthew Marsden, Ph.D.

Lisa Wagar, Ph.D.

Roberto Tinoco, Ph.D.
- Faculty and Staff News
- Media Resources
- Purdue News Weekly
- Research Excellence
- Purdue Computes
- Daniels School of Business
- Purdue University in Indianapolis
- The Persistent Pursuit
- Purdue News on Youtube
- Purdue in the News
- Purdue University Events
The key to fighting viruses: Understanding their structure is vital to unlock a healthy future for humanity

Richard Kuhn, a molecular virologist at Purdue University, works to map out virus vaccines ahead of potential pandemics. (Purdue University photo/Kelsey Lefever)
- Info for journalists
WEST LAFAYETTE, Ind. — There is no cure for the common cold. But there are cures — or at least vaccines — for other viruses, and researchers are working hard to build a toolbox to be able to rapidly develop vaccines for new and emerging viruses.
Viruses are so small that tens of millions of them can fit on the head of a pin. Understanding, let alone fighting, something so infinitesimally small is a crowning challenge of modern medicine.
The physical structure of its molecules dictates how a virus infects people and moves through their bodies as well as how to formulate effective vaccines and treatments.
That physical structure is precisely the expertise of molecular virologist Richard Kuhn , the Trent and Judith Anderson Distinguished Professor in Science in Purdue University’s College of Science and Krenicki Family Director of the Purdue Institute of Inflammation, Immunology and Infectious Disease . He is harnessing that expertise to lay the groundwork for engineering vaccines for a range of viruses.
“The core of what we do is to do analyze and study the structures of viruses and antibodies,” Kuhn said. “We are working to understand what the important features of an antibody are that make it effective at neutralizing — blocking — the virus.
As part of the team that in 2017 mapped the structure of a human antibody that could potently block Zika virus , Kuhn is one of the foremost scouts in the fight against viral diseases.
Viral threats: Tiny entities, big problems
A virus is, arguably, the smallest possible living thing. It is a bundle of DNA or RNA bound up in a minuscule core, sometimes surrounded by a sturdy envelope and sometimes not. It can reproduce, but not by itself. It relies on an outside partner to reproduce: a plant, animal or even a fungus.
In his lab in Purdue’s Department of Biological Sciences , Kuhn studies specifically RNA viruses, particularly those spread by mosquitoes and ticks, including alphaviruses like chikungunya and eastern equine encephalitis, and flaviviruses like Zika, dengue fever, West Nile and yellow fever. His research involves studying the structure of viruses and how they interface with the cells of their hosts’ bodies — hence the mapping of the Zika antibodies.
That structural understanding is key to being able to create vaccines and treatments for viruses.
“With the experience during the pandemic, there is more of a focus on viruses globally,” Kuhn said. “Since the 1970s, at least, there has been a steady stream of known and emerging viruses, including HIV, Ebola and, of course, influenza comes back every year, sometimes with significant effect.”
As climate change increases temperatures and the world becomes more global and interconnected, viruses become more prevalent and have vastly more opportunities to mutate and jump from animal to human hosts. Thus many tick- and mosquito-borne diseases like the ones Kuhn studies are becoming more of an outbreak risk.
The SARS-CoV-2 virus, the cause of the COVID-19 pandemic, is a coronavirus. Before the emergence of SARS-CoV-2, doctors and researchers were already familiar with other coronaviruses and were well on their way to understanding how to build a vaccine for COVID-19. That’s in part why research teams were able to develop effective vaccines so quickly. But all sorts of viruses cause outbreaks and epidemics — not just coronaviruses. Flaviviruses, carried by ticks and mosquitoes, might very well be among the next epidemics humanity faces.
“Despite all the human tragedy, SARS-CoV-2 was a relatively simple target in terms of quickly developing a very effective vaccine,” Kuhn said. “There were features of the virus that made developing a vaccine relatively easy. The next pandemic may not be like that. It’s not going to be like in the movie ‘Contagion,’ where they develop a vaccine in two weeks. The more work we can do now, the more it will pay off in case of a novel pandemic.”
His team may not be crossing the bridge before they come to it, but they’re certainly amassing the timbers and bolts and drawing up the plans.

National Academy of Inventors names two Purdue faculty as 2024 fellows
December 12, 2024

Australian honeycombs abuzz with possibilities for sustainable additive manufacturing

Purdue scientist expecting new world to reveal itself to Mars rover
December 3, 2024

Luna Lu appointed vice president of Office of Industry Partnerships
November 25, 2024
New vaccine platform could aid in fight against deadly viruses
Staff Writer
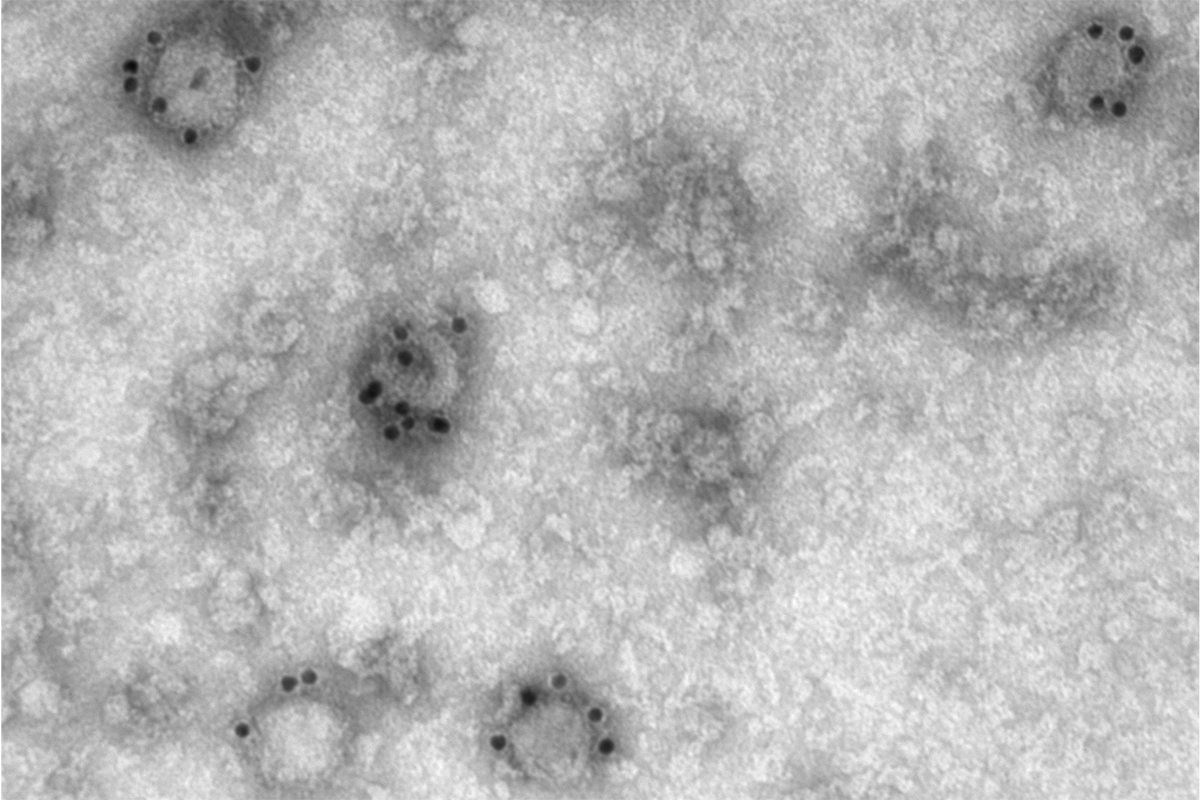
February 17, 2023 – Researchers at Harvard T.H. Chan School of Public Health have developed a new, highly adaptable vaccine platform that could potentially be a powerful tool in the fight against viral pathogens including influenza , HIV , and SARS-CoV-2 .
Postdoctoral fellow Sengjin Choi ; Quan Lu , Cecil K. and Philip Drinker Professor of Environmental Physiology; and others in Lu’s lab created a platform that makes use of tiny particles found in the body called extracellular vesicles, or EVs, which transport various molecules among cells. Working with colleagues at the University of Nebraska, the researchers repurposed a particular type of EVs—called WW domain-activated EVs (WAEVs)—to create the new vaccine platform.
The study was published January 27 in Science Advances.
One common way vaccines work is by using viral proteins as antigens—markers that the immune system can recognize—to stimulate protection against an infection. Antigens are typically proteins or sugars found on the outside of cells or viruses, and each antigen has a unique shape that the immune system can “read” to know whether or not it belongs in the body. If the body recognizes antigens that are dangerous—part of an intruding virus—it will produce antibodies that specifically fit and bind onto those antigens, and neutralize and eventually destroy the virus.
The problem with some vaccines, however, is that the structure of the viral antigens they use may not be exactly the structure needed to kick the immune system into high gear. For instance, certain viruses, such as those that cause the flu, HIV/AIDS, and COVID-19, have antigens that are embedded in a “lipid bilayer”—a double layer of lipids—that coats the virus. Because most current vaccines deliver antigens without a lipid bilayer, the structure of the resulting antibodies may make it more difficult for them to attach to and neutralize the targeted virus.
In the new research, scientists inserted flu or HIV antigens into EVs—which, according to Lu, “are essentially squishy little balls encapsulated with a lipid bilayer,” making them an ideal medium to hold the viral protein antigens in the correct structure so that they can be effectively recognized by the immune system. Testing the EV packages in mouse models of the diseases, the researchers found that the mice that received the EV vaccines had higher antibody production and significantly longer survival rates than those that did not receive those vaccines.
“Our study showed that WAEVs can serve as a novel, highly adaptable vaccine platform that offers a potentially powerful tool in our fight against viral pathogens,” said Lu. “While this study was focused on flu and HIV viruses, the system can be used against other viruses such as SARS-CoV-2.”
Repurposing EVs for vaccine delivery has further public health advantages, Lu said. For example, current mRNA vaccines such as the ones developed for COVID-19 need to be stored in ultra-cold freezer conditions. Because vaccines using EVs are more stable, they only require refrigeration, therefore making it easier and less costly to deliver vaccines to low-resource areas.
– Jessica Lau
Feature photo courtesy Quan Lu
Photo of Quan Lu: Kent Dayton
Related Topics
Last updated, related news.

Malaria transmission may increase with deforestation in the Brazilian Amazon

Artificial intelligence’s potential health benefits, risks discussed at conference
Oct Pre-Prints, Publications and Conferences
Get the latest public health news.
Stay connected with Harvard Chan School
Unleash your potential at Harvard Chan School.
In addition to our degree programs, we offer highly targeted executive and continuing education, directed and taught by Harvard faculty.
Virology Journal
Call for papers, orthoflaviviruses: insights into molecular biology, epidemiology, and control, world aids day, coronaviruses: emerging and re-emerging pathogens in humans and animals.
Orthoflaviviruses, including Dengue, Zika, yellow fever, and West Nile viruses, are transmitted primarily by mosquitoes and ticks, causing significant health issues worldwide. Addressing the global burden of these viruses highlights the critical need for advanced prevention, treatment, and control strategies. Currently open for submissions - Submit Here
Guest Edited by: Professor Olli Vapalahti, PhD, Department Virology, Helsinki University Hospital, University of Helsinki, Finland Professor Denis Kainov, PhD, Department of Clinical and Molecular Medicine, Faculty of Medicine and Health Sciences, NTNU, Norway

For this cross-journal article collection, we welcome submissions from researchers, scientists, clinicians, and academics who are engaged in the scientific study of HIV/AIDS. Currently open for submissions - Submit Here
This thematic series emphasizes advances and key discoveries in the animal origin, viral evolution, epidemiology, diagnostics and pathogenesis of different emerging and re-emerging coronaviruses. Currently open for submissions - Submit Here
Edited by Susanna K. P. Lau, Hayes Luk, Siddharth Sridhar, and Linfa Wang
Global Virus Network - Pandemic preparedness and prevention

Through this partnership, Virology Journal aims to amplify the impact of cutting-edge research by providing a platform for the GVN by publishing a series of commentaries on the theme of pandemic prevention. We look forward to the enriched content and insights this collaboration will bring to our readers and the broader scientific community.
The commentaries can be found here .
Trusted Reviewer Board
Virology Journal is looking to recruit additional reviewers to our Trusted Reviewer Board. The board is open to early-career Virology researchers who would like to gain peer-review experience. Reviewers work closely with the editorial board and will be asked to review one manuscript per month.
Apply here .
Are you, or is someone you know, making a contribution to the UN's SDG3 by working to "ensure healthy lives and promote well-being for all at all ages"? Read our blog post to learn how to raise awareness for your work through our blog series. Applications can be made via our Google Form .
- Most accessed
Evaluation of ALKBH2 and ALKBH3 gene regulation in patients with adult T-cell leukemia/lymphoma
Authors: Yuji Wada, Tadasuke Naito, Takuya Fukushima and Mineki Saito
Risk factors for SARS-CoV-2 pneumonia among renal transplant recipients in Omicron pandemic—a prospective cohort study
Authors: Sai Zhang, Xiang Ding, Chunmi Geng and Hong Zhang
Mechanisms underlying the compromised clinical efficacy of interferon in clearing HBV
Authors: Zhuoyan Lei, Luye Wang, Hanlin Gao, Shubian Guo, Xinjian Kang, Jiajun Yuan, Ziying Lv, Yuxin Jiang, Jinping Yi, Zhi Chen and Gang Wang
Research trends and hotspots on global influenza and inflammatory response based on bibliometrics
Authors: Hui Li, Yanping Zong, Jiajie Li, Zheng Zhou, Yonglong Chang, Weibing Shi and Jinchen Guo
Molecular characterization of circulating infectious bursal disease viruses in chickens from different Egyptian governorates during 2023
Authors: Amr H. Abd El-Fatah, Dalia Ayman, Mahmoud Samir, Soad Eid, Mahmoud Elgamal, A. A. El-sanousi, Mahmoud Ibrahim, M. AlKhazindar, M. M. Ali and Amira Afify
Most recent articles RSS
View all articles
Chloroquine is a potent inhibitor of SARS coronavirus infection and spread
Authors: Martin J Vincent, Eric Bergeron, Suzanne Benjannet, Bobbie R Erickson, Pierre E Rollin, Thomas G Ksiazek, Nabil G Seidah and Stuart T Nichol
Coronavirus envelope protein: current knowledge
Authors: Dewald Schoeman and Burtram C. Fielding
False negative rate of COVID-19 PCR testing: a discordant testing analysis
Authors: Jamil N. Kanji, Nathan Zelyas, Clayton MacDonald, Kanti Pabbaraju, Muhammad Naeem Khan, Abhaya Prasad, Jia Hu, Mathew Diggle, Byron M. Berenger and Graham Tipples
Adverse effects of COVID-19 vaccines and measures to prevent them
Authors: Kenji Yamamoto

The effect of temperature on persistence of SARS-CoV-2 on common surfaces
Authors: Shane Riddell, Sarah Goldie, Andrew Hill, Debbie Eagles and Trevor W. Drew
Most accessed articles RSS
Sign up to receive article alerts
Virology Journal is published continuously online-only. We encourage you to sign up to receive free email alerts to keep up to date with all of the latest articles by registering here .
Latest Tweets
Your browser needs to have JavaScript enabled to view this timeline
Article collections
Discover all the article collections published in Virology Journal . Find out more .
Aims and scope
Virology Journal is an open access, peer reviewed journal that considers articles on all aspects of virology, including research on the viruses of animals, plants and microbes. The journal welcomes basic research as well as pre-clinical and clinical studies of novel diagnostic tools, vaccines and anti-viral therapies.
Journal Sections
Clinical Virology : Fred Kibenge, University of Prince Edward Island, Canada Emerging viruses : Tom Geisbert, University of Texas Medical Branch, USA Hepatitis viruses : Wan-Long Chuang, Kaohsiung Medical University, Taiwan Herpes viruses : Tony Cunningham, The Westmead Institute for Medical Research, Australia Influenza viruses : Hualan Chen, Chinese Academy of Agricultural Sciences, China Negative-strand RNA viruses : John Barr, University of Leeds, UK Other viruses : Erna Geessien Kroon, Universidade Federal de Minas Gerais, Brazil Plant viruses : Supriya Chakraborty, Jawaharlal Nehru University, India Positive-strand RNA viruses : Jaquelline Germano de Oliveira, Fundação Oswaldo Cruz - Fiocruz, Brazil Public health : Kin On Kwok, The Chinese University of Hong Kong Retroviruses : Aguinaldo Pinto, Universidade Federal de Santa Catarina, Brazil Veterinary DNA viruses : Walid Azab, Freie Universität Berlin, Germany Veterinary RNA viruses : James Weger-Lucarelli, Virginia Tech, USA Viruses of microbes : Joana Azeredo, University of Minho, Portugal
Announcing the launch of In Review
Virology Journal , in partnership with Research Square, is now offering In Review. Authors choosing this free optional service will be able to:
- Share their work with fellow researchers to read, comment on, and cite even before publication
- Showcase their work to funders and others with a citable DOI while it is still under review
- Track their manuscript - including seeing when reviewers are invited, and when reports are received

Alan McLachlan, Co-Editor-in-Chief
Alan McLachlan is a molecular geneticist and hepadnavirologist. He currently serves as a Professor in the Department of Microbiology and Immunology, University of Illinois Chicago, USA. His interests are focused on hepatitis viruses, primarily on hepatitis B virus (HBV) and its relationships to liver physiology. His research is currently directed toward understanding the relationships between HBV transcription and viral biosynthesis using both cell culture and animal models. His long-term goals include the identification of cellular gene products as targets for the development of small molecular weight antiviral compounds which, in combination with current nuceot(s)ide analog therapeutics, will resolve chronic HBV infections.

Leo Poon, Co-Editor-in-Chief
Leo Poon is a molecular virologist. He currently serves as a Professor in the School of Public Health, The University of Hong Kong and as a co-director of HKU-Pasteur Research Pole. He has strong interests in emerging viruses, including coronavirus and influenza virus. He researches on different aspects of these viruses, ranging from basic virology to clinical diagnosis. His ultimate goal is to use scientific findings to inform public health policy. Over the years, he has published about 290 peer-reviewed articles. Thus far, his work has been cited over 41,000 times and he has an H-index of 95 (Web of Science).
- Editorial Board
- Manuscript editing services
- Instructions for Editors
- SNAPP Editorial Login
- Contact support for Authors
- Contact support for Editors
- Sign up for article alerts and news from this journal
- Follow us on Twitter
Annual Journal Metrics
Citation Impact 2023 Journal Impact Factor: 4.0 5-year Journal Impact Factor: 3.8 Source Normalized Impact per Paper (SNIP): 0.971 SCImago Journal Rank (SJR): 1.016
Speed 2023 Submission to first editorial decision (median days): 8 Submission to acceptance (median days): 109
Usage 2023 Downloads: 2,323,860 Altmetric mentions: 11,783
- More about our metrics
ISSN: 1743-422X
- Submission enquiries: [email protected]
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List
RNA Viruses: RNA Roles in Pathogenesis, Coreplication and Viral Load
Palmiro poltronieri, binlian sun, massimo mallardo.
- Author information
- Article notes
- Copyright and License information
Address correspondence to this author at the Department of Molecular Medicine and Medical Biotechnology, University of Naples Federico II, Via S. Pansini 5, 80131 Napoli, Italy; Fax: +39-081-7463205; E-mail: [email protected]
Received 2015 Mar 28; Revised 2015 Apr 10; Accepted 2015 Apr 14; Issue date 2015 Oct.
This is an open access article licensed under the terms of the Creative Commons Attribution-Non-Commercial 4.0 International Public License (CC BY-NC 4.0) (https://creativecommons.org/licenses/by-nc/4.0/legalcode ), which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
The review intends to present and recapitulate the current knowledge on the roles and importance of regulatory RNAs, such as microRNAs and small interfering RNAs, RNA binding proteins and enzymes processing RNAs or activated by RNAs, in cells infected by RNA viruses. The review focuses on how non-coding RNAs are involved in RNA virus replication, pathogenesis and host response, especially in retroviruses HIV, with examples of the mechanisms of action, transcriptional regulation, and promotion of increased stability of their targets or their degradation.
Keywords: Argonaute, DICER, HIV, RNA binding proteins, RNA secondary structure, RNA viruses, Small RNAs.
1. INTRODUCTION
RNA agents have been shown to play essential roles in evolution and regulation in all DNA/protein based life: based on RNA stem-loop secondary structures (built of paired stems and not-paired loops), pseudoknots, and loops with sequences showing affinity to target proteins. Group I and group II introns, viroids, viral (RNA and DNA viruses, bacteriophages, retrotransposons, Long Terminal Repeats) networks cooperate within cellular genomes as modular. Some non-coding RNAs have built complementary consortia, such as rRNAs, tRNAs, spliceosomes, editosomes, and other ribonucleoprotein particles (RNPs) [ 1 ]. Additionally, counterbalancing modules such as restriction/modification (RM) modules have evolved, assuring identity (self/non-self) of organisms. All fine-tuned steps of key cellular processes such as gene expression, transcription, translation, DNA recombination and repair, epigenetic imprinting, as well as various forms of innate and adaptive immunity, are essentially constituted by natural genetic content operators.
There are many reports showing that virus infection can alter the cellular microRNAs (miRNAs) to affect virus replication, some of them oppose the function of host restriction factors to enhance virus proliferation [ 2 , 3 ]. Nowadays, researchers are trying to design miRNAs against virus proteins to control virus replication.
In the organisms, RNAs are associated to RNA binding proteins, helicases and RNases involved in RNA degradation and turnover.
This review discusses how these RNA interacting proteins and networks of regulatory RNAs are integrated intothe general network of pathogenesis’ control, especially during RNA virus infection.
1.1. RNA Viruses
Human diseases causing RNA viruses include Orthomyxoviruses, Hepatitis C Virus (HCV), Ebola disease, SARS, influenza, polio measles and retrovirus including adult Human T-cell lymphotropic virus type 1 (HTLV-1) and human immunodeficiency virus (HIV). RNA viruses have RNA as genetic material, that may be a single-stranded RNA or a double stranded RNA. Viruses may exploit the presence of RNA-dependent RNA polymerases for replication of their genomes or, in retroviruses, with two copies of single strand RNA genomes, reverse transcriptase produces viral DNA which can be integrated into the host DNA under its integrase function. Studies showed that endogenous retroviruses are long-terminal repeat (LTR)-type retroelements that account for approximately 10% of human or murine genomic DNA.
Among human retroviruses, HIV-1 is a lentivirus with an RNA genome formed by two copies of a single-stranded, positive-sense RNA. The HIV-1 RNA genome is associated to the nucleocapsid protein (NC) and to viral enzymes, thus it is “protected” within the viral capsid mainly formed by the p24 protein. Upon entry into the target cell, the viral RNA genome is reverse transcribed into double-stranded DNA by a virally encoded reverse transcriptase that is transported along with the viral genome into the virus particle. The viral DNA is imported into the cell nucleus and integrated into the cellular DNA by a virally encoded integrase and host co-factors. Once integrated, the virus may become latent, or may be transcribed, producing new RNA genomes and viral proteins that are packaged and released from the infected cell as new virus particles that will infect other cells to begin the new replication cycle. Many aspects of the life cycle of retroviruses are intimately linked to the functions of cellular proteins and RNAs. HIV-1 and Moloney Murine Leukemia Virus (MoMuLV) have been studied for the dimerization of two RNAs.
1.2. Retrotransposons, LTRs, Retrotranscriptases
Long Interspersed Nuclear Elements-1 (LINE-1) and endogenous retroviruses (HERVs) encode reverse transcriptase (RT) proteins in vertebrates. LINE-1s (L1s), the most studied, active autonomous mobile DNA in humans, accounts for about 17% of human DNA while HERVs account for about 8% and, together with the non-autonomous SINE/Alu family (about 10%) constitute a large proportion of the human genome. L1s encode two open reading frames (ORFs 1 and 2). The shorter ORF1 translation product (ORF1p) is an RNA binding protein, thought to also bind to non-retroviral transcripts, protects against nuclease degradation and specify nuclear import of the ribonuclear protein complex (RNP). ORF2 encodes a multifunctional protein (ORF2p) comprising apurinic/apyrimidinic endonuclease (APE) and reverse-transcriptase (RT) activities, responsible for retroelement’s replication and their integration into chromosomal DNA. However, some clades of APE-type retroelements only encode a single ORF-corresponding to the multifunctional ORF2p [ 4 ]. HERVs, closely resembling infectious retroviruses, have mutated and/or truncated provirus structures and have lost their ability to replicate or retrotranspose. Nonetheless, proteins encoded by different types of HERVs are still exerting biological activities and most of the HERV-associated regulatory regions, termed “long terminal repeats” (LTRs), preserve their functions as a promoter–enhancer region. Functional “awakening” of HERVs and LTRs from their epigenetic silencing can play causative roles in tumorigenesis; in particular, HERV-K (HML-2), the most recently integrated family with a nearly complete retroviral structure, is involved in neoplastic and autoimmune pathological processes [ 5 ]. Retroelements, which mobilize throughout the genomes by a copy-and-paste process involving RNA intermediates, have the potential to modify mammalian genomes not only through insertional mutagenesis yet generating many other novelties that alter genomes both structurally and functionally [ 6 ]. Not surprisingly, cells have adopted strategies aiming at restricting the mobility and deleterious consequences of uncontrolled retrotransposition [ 6 ].
Although heavily mutagenic and responsible for deleterious gene disruptions, retroelements may have provided some beneficial genomic functions with potential evolutionary advantages.
Incubation of mouse zygotes with 5′-bromodeoxyuridine (BrdU) yields massive incorporation of this nucleoside analogue in newly synthesized DNA; surprisingly, a significant incorporation still occurs in both zygotic pronuclei in the presence of aphidicolin, a specific inhibitor of DNA replication. This aphidicolin-resistant BrdU incorporation is quantitatively abolished when embryos are simultaneously exposed to abacavir, a nucleoside RT inhibitor, thus revealing its RT-dependent nature. Moreover, quantitative PCR analysis showed that LINE-1 copies are newly synthesized at the zygote- and two-cell embryo stages and nearly doubled compared to gamete copy number. These findings support the conclusion that RT-dependent amplification of LINE-1 retrotransposons is a distinctive feature of early embryonic genomes [ 7 ].
Inhibition of RT activity in cancer cell lines, either by LINE-1-specific RNA interference or by RT inhibitory drugs, was found to reduce proliferation and promote differentiation and to antagonize tumor growth in animal models. Using CsCl density gradients, Alu- and LINE-1-containing DNA:RNA hybrid structures were identified in cancer yet not in normal cell lines [ 8 ]. In cancer cells the highly abundant RT activity intercepts various RNA classes and reverse transcribes them generating RNA:DNA hybrids. This may impair the formation of double-stranded RNAs required in the production of small regulatory RNAs (miRNA in particular), with a direct impact on gene expression. RT inhibition restores the 'normal' small RNA profile and the regulatory networks that depend on them. Thus, the retrotransposon-encoded RT drives a previously unrecognized mechanism crucial to the transformed state in tumor cells.
Recent computational studies confirm the association between L1 expression and the generation of small RNAs. L1 expression seems to have a role in the activation of small RNA expression as emerged comparing data from L1-active and L1-silenced breast cancer cells. Cells in which L1 expression was silenced greatly increased the expression of a number of miRNAs, in particular members of the let-7 family, few piwiRNAs and several repeat-RNAs targeting LTRs, LINEs and SINE elements [ 9 ].
1.3. Small RNAs, miRNAs, RNA Interference and Immunity
miRNAs have become a prototype of several classes of small RNAs (sRNAs) [ 10 ]. These sRNAs act as single strand filaments incorporated into the RNA induced silencing complexes (RISC) acting as guides to Argonaute (Ago) enzymes degrading the target RNA complementary to these sRNAs. The processing into mature sRNAs requires protein complexes containing endoribonucleases: DROSHA in the nucleus, cleaving the dsRNA precursors into pre-miRNAs. The pre-miRNA hairpin structure is then exported into the cytoplasm via exportin-5. In the cytoplasm it assembles in RNA induced silencing complex (RISC) which includes Dicer ribonucleases that cleave pre-miRNAs into mature single strand sRNAs, Ago enzymes cleaving the mRNAs with sequences complementary to the microRNAs [ 11 ], and other associated proteins, such as PW182/Argonaute-2, a 182 kDa RNAse associated to P-bodies [ 12 ], HIV transactivating response RNA-binding protein (TRBP) [ 13 ], and fragile X mental retardation protein (FMRP1) [ 14 ]. Mature miRNAs play important roles in the regulation of mammalian genes. It has been suggested that over 30% of all human genes are regulated by miRNAs. While recent genome-wide siRNA and shRNA screenings have shown that several hundred host cell proteins contribute to the regulation of HIV-1 infection in human cells, how miRNA-mediated regulation complements this picture is poorly understood. Other processing complexes are involved in the production of sRNA with various sizes: piwiRNA and siRNA, with size ranging from 21-nt to 25-nt, is the output of this processes. Their role is in the amplification of signals such as RNA primed RNA amplification, and the spreading of anti-viral RNA interference mechanism.
1.4. RNA Pseudoknots, Hairpins and Secondary Structures
RNA can both store information in its linear sequence and take on critical structural and catalytic roles in the cell, such as during the translation of messenger RNA into proteins [ 15 ]. These latter functions depend on the complex higher-order structures RNA is able to form. Homan et al. reported a method to probe the intricate conformational states in the analysis of HIV-1 NL4-3 RNA genome [ 15 ]. They chemically modified exposed segments of three complex RNA structures. They then sequenced the RNA to map the locations of the multiple modifications in each individual linear RNA molecule. This allowed the researchers to deduce interactions in three-dimensional space, and to uncover the local conformation, providing valuable information on the folding and function of RNAs [ 15 ]. Single-molecule RNA structure was tagged, i.e. multiple sites were chemically modified are identified by massively parallel sequencing of single RNA strands, and then analyzed for correlated and clustered interactions. The strategy thus identified RNA interaction groups by mutational profiling (RING-MaP) and made possible two applications. Firstly, through space interactions, 3D models were created for RNAs, spanning 80–265 nucleotides, and intramolecular interactions that stabilize RNA were characterized. Secondly, distinct conformations in solution were identified and revealed previously undetected hidden states and large-scale structural reconfigurations that occur in unfolded RNAs relative to native states. RING-MaP analysis of single-molecule nucleic acid structure enabled a novel view of the global architecture and multiple conformations that govern the functions in RNAs.
Additional methodologies that have been used in testing the secondary structure of RNA genomes have been published. 2’-hydroxy acylation of RNA was analysed by primer extension and mutational profiling (SHAPE-MaP) [ 16 ] and used to define a new model of HIV-1 RNA genome.
Advances in RNA structure prediction from sequence are currently made by setting and testing new tools for generating hypotheses and confirming viral RNA structure-function relationships [ 17 ]. On this basis, novel methods have been tested to investigate the sequence-dependence of RNA-protein interactions [ 18 ]. RNA substrates demonstrate diverse intramolecular interactions, including mismatched base bulges, stem loops, pseudoknots, g-quartets, divalent cation interactions and noncanonical base pairs, determining three-dimensional RNA structure. The molecular evolution of MS2 from low- to high-affinity hairpins, was analysed and quantified. The results suggest that quantitative analysis of RNA on a massively parallel array (RNA-MaP) provided an insight into the biophysics of RNAs and on consequences of sequence-function relationships.
Several RNA secondary structures have been shown important for the virus functions: internal ribosomal entry structure, internal ribosomal entry site, and 5' UTRs regulate the start of translation of operons. For example, in influenza virus type C, there are seven vRNA segments with non-coding regions (NCR) at the extremities, that affects transcription and replication, by the type-C and type-A polymerase complexes [ 19 ]. To determine the molecular structure adopted by these NCR, various bioinformatics tools, including RNAfold, RNAstructure, Sfold, and Mfold, have been used. Various nucleotide polymorphisms (SNPs) in these non-coding regions may differentiate infective strains, such as major or minor read-through activity and differential expression of ORFs in operons. In Orthomyxoviridae, such as human influenza viruses or infective salmon anemia virus (ISAV), studies suggest an association between the molecular architecture of NCR regions and their role in the viral life cycle [ 20 ]. The 3' and 5'-terminal sequences of influenza A, B and C virus RNA segments are highly conserved and show partial inverted complementarity [ 21 ]. The viral RNA 3’- and 5’-end structure and mRNA transcription of infectious salmon anaemia virus resemble those of influenza viruses [ 22 ]. The aligned Non-Coding Region (NCR) sequences from ISAV isolates were compared with those from influenza virus, and consensus sequences were found, based on conserved regions identified in the consensus sequence [ 23 ]. This hypothetical structure, together with a comparison with influenza viruses, yielded reliable secondary structure models that lead to identification of conserved nucleotide positions at inter-genus level to determine which nucleotide positions are involved in the recognition of the vRNA/cRNA by RNA-dependent RNA polymerase (RdRp) or mRNA by the ribosome. The NCR contain conserved sequences that vary in length among the various genera of the family Orthomyxoviridae [ 24 ]. It has been reported that the first 12 and 13 nucleotides correspond to conserved sequences in the 3’ and 5’ ends, respectively, of all segments of the influenza A vRNA [ 25 ]. Structurally, these conserved sequences in influenza A have been described as partially complementary and capable of interacting in cis within each segment of RNA, forming structures called panhandles [ 26 , 27 ] In Orthomyxoviruses, transcription of the genome requires the vRNA to act as template for each genomic segment, and for transcription to occur, the conformation adopted via the folding of the NCR is essential [ 26 ].
1.5. HIV-1: Host Factors Supporting From the Entry to Virus Replication
Recently importin 7 and importin α have been shown to enhance nuclear entry of HIV-1 (but not HIV-2) correlating with its ability to bind to the viral integrase and the virus accessory protein Vpr which are components of pre-integration complex (PIC) [ 28 , 29 ]. Transportin 2, identified using siRNA screens [ 30 - 32 ], is also able to enhance nuclear import of PIC. The most important finding was that tRNA molecules themselves can act as nuclear entry chaperones for the HIV PIC. HIV-1 transcription is regulated by the viral promoter located in the 5′LTR of the provirus. The LTR contains binding sites for several transcription factors such as Sp1 and NF-κB, NFAT, LEF-1, COUP-TF, Ets1, USF and AP-1 [ 33 ].
The RNA binding protein Staufen appears to act as a chaperone to the RNA and has been detected in viral particles. Similarities between this and the known HIV TAR RNA binding protein TRBP may promote further investigations.
RNA cap methylases are cellular factors that regulate post transcriptional HIV-1 RNA expression in order to produce a viral mRNA camouflaged by cellular mRNA showing a 7-methylguanosine (m7G) cap.
1.6. RNA Silencing and Host-virus Interaction
Plants and lower eukaryotes produce miRNAs and siRNAs as a form of RNA-interference (RNAi) to restrict infecting viruses. While mammals conserve the same functional miRNA repertoire and RNA-silencing machinery, some have debated whether they employ a miRNA-based antiviral strategy. For endogenous mammalian retroviruses, there is a large body of literature demonstrating that a variety of small non-coding RNA forms are employed to silence these elements. In silico analyses have also indicated that exogenous mammalian viruses may be similarly susceptible to miRNA-based restriction. The notion that miRNAs restrict viruses in mammals as they do in invertebrate or plant cells is supported by increasing examples of RNAi-silencing suppressors encoded by mammalian viruses such as Adenovirus, HCV, Ebola, Influenza A virus, primate foamy virus, HIV, SARS corona virus and HTLV-1. Further investigation is needed to understand how RNA-based and protein-based viral restriction mechanisms cooperate together in human cells.
Considering tumour- associated viruses (oncoviruses), it is estimated that 20% of all cancers are linked to infectious agents. Studies of oncogenic DNA viruses have contributed to the understanding of key molecular mechanisms of tumorigenesis and viral oncogenicity [ 10 ]. Virally encoded oncoproteins such as adenovirus E1A and human papillomavirus (HPV) E7 can bind an array of cellular proteins to override proliferation arrest. Adenovirus VA1 noncoding RNA can inhibit small interfering RNA and microRNA biogenesis, both by inhibiting nuclear export of shRNA or pre-miRNA precursors, competing for the Exportin 5 nuclear export factor, and inhibiting Dicer function by direct binding to Dicer [ 10 ]. Recently, many viral-encoded miRNAs have been discovered, especially abundant in viruses transcribed from double-stranded DNA genomes. Several virus-encoded miRNAs have unique aspects to their biogenesis, such as the location within the precursor transcript.
Bovine leukemia virus, a member of the retrovirus family, was the first RNA virus shown to synthesize a viral RNA that is proficiently processed in cells into small ncRNAs [ 34 ], producing numerous miRNAs. BLV avoids Drosha-mediated cleavage of its genome and mRNAs, which overlap the miRNA cluster, since BLV miRNAs, unlike most known miRNAs, are encoded as shorter RNA polymerase III (pol III) transcribed hairpins that can directly serve as Dicer substrates. Thus, BLV transcripts are not cleaved by Drosha, while subgenomic small RNAs are processed into miRNAs.
Influenza A virus replicates its genome in the nucleus and is exposed to the nuclear microRNA processing factors Drosha and DGCR8. At 8 hours after infection, 18- to 27-nt small viral leader RNAs (leRNAs) bearing a 5'-terminal triphosphate are produced from the 5' ends of all eight influenza virus genomic RNA (vRNA) segments [ 35 ]. The high-level production of leRNAs may imply a role in the regulation of the switch from viral mRNA transcription to genomic RNA synthesis.
It is generally believed that cytoplasmic RNA viruses do not encode miRNAs, owing to inaccessible cellular miRNA processing machinery. In a genome-wide analysis and identification of miRNAs originating from hepatitis A virus (HAV), a typical cytoplasmic RNA virus [ 36 ], two novel virally encoded miRNAs, hav-miR-1-5p and hav-miR-2-5p, were identified, generated from viral miRNA precursors (pre-miRNA).
Presently, functions have been proposed for viral miRNAs from three different viral families: herpesviruses, polyomaviruses and retroviruses.
Four vsRNAs were detected in enterovirus 71-infected cells using next-generation sequencing and northern blots. Viral infection produced substantial levels (>10 5 copy numbers per cell) of vsRNA1, one of the four vsRNAs. Dicer was shown to be involved in vsRNA1 generation in infected cells. vsRNA1 overexpression inhibited viral translation and IRES activity in infected cells. Conversely, blocking vsRNA1 enhanced viral yield and viral protein synthesis. vsRNA1 targets stem-loop II of the viral 5' untranslated region and inhibits the activity of the IRES through this sequence-specific targeting [ 37 ].
Websites and databases are available that classify most of the small RNAs and mRNAs produced by virus families [ 38 ]; In VIRMir database four miRNAs are recorded derived from HIV genome hiv1-miR-tar-5p, hiv1-miR-tar-3p, hiv1-miR-n367, originating from nef gene and targeting nef mRNA [ 39 ], hiv1-miR-h1 [ 40 ]. In addition to these four, another one, hiv1-miRH3, was reported more recently [ 41 ], which locates in the mRNA region encoding the active centre of reverse transcriptase (RT), targets HIV 5’-LTR and binds to the TATA box, upregulating promoter activity [ 41 ].
The HIV-1 Trans-Activation Response (TAR) element is a hairpin structure of ~50 nucleotides found at the 5' end of the HIV viral mRNA. TAR element is recognized by the RNAi machinery and it has been shown to be processed by Dicer yielding a viral miRNA involved in chromatin remodelling of the viral LTR [ 42 ] and targeting the apoptosis genes ERCC1 and IER3 [ 43 , 44 ]. This viral miRNA is detectable in infected cells and appears to contribute to viral latency.
In a recent publication [ 45 ] numerous small RNAs were found deriving from HIV-1 RNA genome. Most of the sequences, with positive polarity (98.1%) could be structured RNAs (sRNAs) or miRNA-like (vmiRNAs). A small portion of the viRNAs, with negative polarity, is encoded within the 3′-UTR. These viral siRNAs (vsiRNAs) were shown to act inhibiting virus replication, since their inhibition using antagomiRs increases virus replication. Three of the HIV-1 small RNAs were shown to be processed by the RNAi machinery. There are data showing that HIV-1 can express an antisense transcript from the 3'-end of its genome that forms long RNA duplexes with counterpart sense HIV-1 RNAs [ 46 - 48 ]. Most HIV sRNAs are not supposed to function as miRNAs, because of lack of evolutionary conservation amongst strains, but may still assume a hairpin structure in the regions containing the conserved bases.
1.6.1. Host miRNAs Deregulated by Virus Infection
Presently, viral interactions with cellular miRNAs have been identified, expanding the knowledge of miRNA functions [ 49 ].
One of the first host miRNAs shown to block retrovirus was miR-32, effectively limiting primate foamy virus type 1 (PFV-1) replication [ 50 ].
Inhibition of influenza virus replication has been described for four miRNAs: miR-323, miR-491, miR-654, and let-7c. Vesicular stomatitis virus is inhibited by miR-24 and miR-93, hepatitis B virus by miR-125a-5p, miR-199a-3p and miR-210; and HCV by miR-196, miR-296, miR-351, miR-431, and miR-448 [ 51 ]. In the case of HCV, a liver-specific miRNA, miR-122, was found to directly target HCV RNA sequence to up-regulate viral replication [ 52 ].
Substantial advances have been made in the understanding of the interplay between HIV-1 and the cell's RNAi activity. HIV-1 infection can change the miRNA expression profiles in the circulating blood cells from infected individuals [ 51 ].
Host miRNAs can modulate HIV replication either directly by targeting HIV RNA, or targeting the mRNAs that encode host cell factors relevant to HIV replication. miR-217 was found induced by Tat and increased HIV-1 expression by targeting sirtuin-1 (SIRT-1) with deacetylase activity inactivating Tat function [ 53 ]. miR-198 was shown to inhibit HIV-1 gene expression and replication in monocytes, action linked to its down-regulation of cyclin T1 [ 54 ].
Recent reports studied several human miRNAs targeting HIV-1 sequences. Using target prediction software, five miRNA (miR-29a, miR-29b, miR-149, miR-324-5p, and miR-378) were found to target sequences, two of them located in the viral nef gene, of the HIV-1 genome [ 55 ]. miR-29a was shown to inhibit nef expression, and to repress HIV replication in Jurkat cells. Recently inhibition of HIV-1 infection by miR-29a and miR-29b was confirmed [ 56 , 57 ], however, HIV-1 is protected by a complex RNA secondary structure surrounding the target site. A different group of five miRNAs (miR-28, miR-125b, miR-150, miR-223, and miR-382) that target the 3'-UTR of the HIV genome was reported [ 58 ]. These “anti-HIV” miRNAs were shown to be enriched in resting CD4+ T cells and were hypothesized to be involved in proviral latency. In another study, four of these miRNAs were found responsible for differences between monocytes and macrophages in their permissivity to HIV infection [ 59 ]. Recently the action of miR-29, miR-133b, miR-138, miR-149 and miR-326, targeting HIV-1 sequences, was shown [ 60 ]. Therefore, in divergent cells and in varying contexts different miRNAs may selectively regulate HIV-1 infection through direct targeting viral sequences. Thus, a complex set of miRNA-mediated positive and negative regulatory events is influencing viral replication [ 51 ]. In monocytes, miR-1236 was shown to inhibit HIV-1 infection by repressing translation of cellular factor Vpr binding protein, VprBP/DCAF1 [ 61 ].
A significant number of host non-coding RNAs have been found in Hepatocellular carcinoma (HCC) caused by HCV infection, and are involved in pathogenesis of HCV and HCV-induced HCC [ 62 ].
1.7. Subversion of IFN Responses
The role of type I-IFNs in increasing host susceptibility could be explained by modulation of components of the immune response involved in controlling the growth of infective agents, such as induction of T cell apoptosis, resulting in greater IL-10 secretion by phagocytic cells, in turn dampening the innate immune response. A mechanism by which viruses survive inside cells is by inactivating the cellular antiviral machinery, or inactivating the RNA interference response, acting on the dsRNA-activated protein kinase (PKR). Infection thus can escape from the immune response by deregulation of the interferon signaling and the processes forming small RNAs acting in RNA silencing pathways.
1.8. Virus Deregulation of Stress Granule Function
It was shown that viral life cycle within cells involves hijacking cellular processes and nuclear targeting. This is also at the base of redistribution of translation machinery during the stress response involving the formation of stress granules, processing bodies (P-bodies, PB), and perinuclear paraspeckles. During oxidative stress, arsenite, or by phosphorylation of eIF2α, cells undergo a translation arrest, stalling the RNAs in the form of ternary complexes that include eukaryotic initiation factor 2 (eIF2)/GTP/Met-tRNA. These stress granules, containing the RNA to be translated, have a role in spatial and temporal inhibition of mRNAs, until resolving the stress for processing the mRNAs, or degrading it in case of non recovery from the stress. Stress granules are formed by a nucleation process that involves several principal factors, such as the RNA decay factor G3BP, which prevents the localisation of ribosome and initiation factors in silenced SG foci, the translational suppressor TIA1, TIAR, Caprin1, USP10, DDX6 (Rck/p54), DDX3 helicase, poly-A binding protein PAPB and Lsm1. Additionally, SGs contain enzymes of the RNA silencing pathway, such as Argoanute-2, trans-acting factors, Hsp90 complexes and RNA binding proteins, found at the site of small RNA-mediated repression of RNA targets [ 63 ].
Different viruses exploit the binding to protein scaffolds to avoid SG formation or to assemble their RNA into SGs devoid of cellular RNA, thus exploiting the transcriptional machinery for their own means [ 64 ]. In RNA viruses, the knowledge has increased recently, especially focusing on HCV infected cells, where SG and P-body components are relocalised to the periphery of lipid droplets, and an oscillation between SG assembly and disassembly is observed upon interferon I treatment, depending on the inhibition of PKR by the eIF2 phosphatase GADD34 [ 65 ]. West Nile Virus (WNV) inhibits SG formation by scavenging Reactive Oxygen Species (ROS), and also relocalising the SG scaffolding proteins into perinuclear foci where WNV replication occurs by exploiting cell translation machinery. HIV-1 blocks SG assembly in vitro and ex vivo in patient samples. Gag has an important role in inhibition of SG, dependent on the interaction between host factors EIF2 and G3BP1. Influenza A virus (IAV) proteins can block SG formation: IAV polymerase complexes function in the nuclei of infected cells, generating mRNAs with a 5’ cap and polyA-tail that are transferred into the cytoplasm for translation. Non-structural protein 1 (NS1) inactivates PKR, preventing eIF2 phoshorylation; nucleoprotein (NP) inhibits SG formation through eIF independent mechanisms; host-shutoff protein polymerase-acidic protein-X (PA-X) is essential to block SG formation. Measles virus infection progresses through the synthesis of 5’-copyback defective-interfering RNA (DI-RNAs), that are complementary in the 5’ and 3’ termini, forming double stranded RNAs, efficient in activation of PKR and PKR signaling. Downstream to this event, measles protein C is required for alleviation of SG translation inhibition, while A-to-C mutation events dependent on ADAR modify the virus genome. In picornaviruses, such as enteroviruses, proteinases have been shown involved in disassembly of SG, while in kobuviruses other factors, such as a small leader peptide, are important in SG inhibition.
1.8.1. Vpr/Vpx
The viral accessory protein Vpr is a component of the PIC. It is reported that Vpr is important on the nuclear import of PIC by interacting with a nuclear pore protein, importin α [ 29 ]. Since PIC is larger than a nuclear pore, various other nuclear pore complex proteins have also been identified in PIC nuclear entry, including Nup 98, Nup 124p, Nup 358 and Nup 153.
Vpr belongs to the RAD23-like family of proteins, similarly to Vpx [ 66 ]. Vpr is a chaperone that guides target proteins to bind to VprVP/DCAF1, a receptor of the CULLIN E3 ubiquitin ligase (Cul4-DDB1, Cul5) [ 67 - 69 ]. Vpx is a small virion-associated adaptor protein encoded by viruses of the HIV-2/SIVsm lineage of primate lentiviruses, a Vpr paralogue, that enables these viruses to infect monocyte-derived cells.
One of the main activities of Vpr/Vpx is the degradation of target proteins through binding to VprBP, thus recruiting the 26S proteasomal pathway. Several studies showed the potentiality of Vpr to interact with many E2 and E3 enzymes [ 70 ]. Vpr has been shown to affect, either directly or indirectly, the modification of proteins, such as ubiquitinylation, phosphorylation and neddylation. In this way, Vpr influences and regulates the levels of many proteins [ 71 ].
Vpr has been found to affect the levels of few miRNAs among which is miR-34a, as well as the genes IRBIT, SERP1, SIRT1, NEFM, Drp-1, Orai, STIM1, IP3R and CREB [ 72 ]. It was reported that Vpr inhibits short hairpin RNA function as expected upon reduction of endoribonuclease Dicer levels by binding with VprBP to block maturation of miRNAs [ 73 ].
Also it was reported that Vpr can interact with spliceosomal protein SAP145 to mediate cellular pre-mRNA splicing inhibition. Although the mechanism is not clarified, Vpr is sufficient alone to promote HCV RNA replication [ 74 ]. Vpr is reported to be a component of the reverse transcription complex (RTC) and co-localizes with the viral nucleic acid and integrase within purified HIV-1 RTCs.
Vpr also can regulate several proteins and host factors, some of them can affect RNA replication. The most interesting are TERT [ 67 ], the type I interferon regulatory factor 3 (IRF-3) [ 75 ], A3G [ 76 ], TRIM proteins [ 77 ], uracil DNA glycosylase 2 (UNG2) [ 78 ] and single strand selective monofunctional uracil-DNA glycosylase 1 (SMUG1). A premature activation of the SLX4 complex has been shown dependent on Vpr, promoting G2/M arrest and escape from innate immune sensing [ 79 ].
Zahoor et al. using microarray system found that Vpr protein enhanced the mRNA level of interferon (IFN)-stimulated genes (ISGs), and causes phosphorylation of STAT1 at tyrosine 701 in human monocyte-derived macrophages (MDMs) infected with a recombinant adenovirus expressing Vpr [ 80 ]. These findings enhance the current understanding of HIV-1 replication and pathogenesis in human macrophages. Vpr, together with other HIV factors, recruits cellular adaptors to facilitate immune evasion [ 81 ]. HIV-1 Vpr differentially regulates the expression levels of chemotactic cytokines such as CXCL1, CXCL5, CXCL7, CXCL9, CXCL10, and CXCL11. A report showed that CXCL10 and CXCL11 are up-regulated in HIV-1-infected macrophages and play a key role in the recruitment and spread of HIV-1 to susceptible CD4+ T-cells [ 82 ].
1.9. Intracellular Defences Against HIV
Clearly the cell is not a passive participant in virus replication. In addition to the cellular pathways subverted by the virus for its own use there are inhibitory factors within cells which act as intracellular defences and whose presence inhibits or ‘restricts’ the virus. The first one, identified in retrovirus infected cells, was Fv1 which restricts ecotropic murine leukemia viruses. Following this finding, other similar factors restricting HIV were identified. Because of their potential importance in novel antiviral approaches, they have been extensively investigated in recent years. APOBEC3 family [ 83 ], TRIM family [ 77 , 84 ], Tetherin [ 85 ], IRF3 [ 75 ], SMUG1, UNG2 [ 86 ], SAMHD1 [ 87 ] and SLX4 [ 79 ] were well known for being involved in HIV infectivity, indicating that the viral proteins can interact and modulate their activity.
As an adaptive response, viruses develop the ability to interact and deactivate these defences, a mechanism named pathogen mimicry. Among several mechanisms, there are: a) the development of proteins and molecules that act interfering with cellular processes; b) virus miRNA analogs of host miRNAs, exploiting the presence of a network of cell effectors and antiapoptotic factors; c) incorporating protein-protein interaction domains or association modules in their genome; d) through increased mutation rates evolving the recognition domains of proteins targeted by cellular defences.
1.9.1. Epigenetics. Resetting of Epigenetic Marks
Infective agents and bacteria when entering inside the cells activate several mechanisms to avoid immune detection [ 84 ]. Many viruses entering inside the cells are able to derail the cellular machinery, including the epigenetic control. A large set of host proteins required for HIV infection have been identified through a functional genomic screen [ 88 ]. RNAi screens have been performed for host factors required for HIV replication [ 30 ].
HIV-1 integration is generally random but it has more easier access into active genes; however, independent of the site of integration in human chromosomes, two nucleosomes, named nuc-0 and nuc-1, are precisely organized in the 5′LTR. In particular, the histone organized nuc-1 structure (located at position −2 to +140 of the LTR) normally serves to down modulate basal transcription.
The HIV-1 transcriptional activator Tat has evolved mechanisms to resolve the transcription block. Tat is associated with histone acetyl transferase (HAT) proteins whose activities remodel nucleosomes to allow transcriptional access. Tat has been shown to bind several different HATs: CBP/p300, p/CAF, GCN5, Tip60, and TAFII250. Through binding to the HAT proteins, Tat relieves chromatin repression at the HIV-1 LTR. Recently, Tat has also been found to bind a histone chaperone protein, hNAP-1, which acts with ATP-dependent chromatin remodeling complexes to facilitate transcription.
Counteracting the effect of HATs, the histone deacetylase proteins (HDAC) remove the acetyl-group from HAT-acetylated histones to enforce transcriptional silencing. In the HIV-1 LTR, it is thought that the LSF protein binds at position −10 to +27 of the LTR to recruit the YY1 factor which further binds HDAC-1 to silence viral transcription. Tat expression down regulates HDAC-1 to remove the transcription repression. A similar recovery from repressive inhibition has been obtained through treatment with HDAC inhibitors (HDACIs) such as Trichostatin A (TSA), Valproic Acid (VPA), and sodium butyrate.
1.10. Conclusion
In this review we highlighted the importance of cellular and viral RNAs in the cell response to RNA viruses, especially to retroviruses and endogenous L1 remnants of viral DNA integration. In addition, we reviewed several pathways involving small RNAs and short interfering RNAs deregulated in various states, from active infection to virus-associated cancers and defective immune signaling. A special role has been assigned to the deregulation of interferon response and the inhibition of protein complexes in stress granules and P-bodies, RNA binding proteins, RISC components and the RNA silencing machinery.
ACKNOWLEDGEMENTS
PP was supported by the national project PON 02_0186_3417512 Strumenti Innovativi per il Miglioramento della Sicurezza Alimentare (S.I.MI.S.A.). BS was supported by the National Science Foundation of China (31470270).
We thank Dr. Concetta Ambrosino and Dr. Giuseppe Fiume for the critical reading of the manuscript.
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflict of interest.
- 1. Villarreal L.P., Witzany G. Rethinking quasispecies theory: From fittest type to cooperative consortia. World J. Biol. Chem. 2013;4(4):79–90. doi: 10.4331/wjbc.v4.i4.79. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 2. Sullivan C.S., Ganem D. MicroRNAs and viral infection. Mol. Cell. 2005;20(1):3–7. doi: 10.1016/j.molcel.2005.09.012. [ DOI ] [ PubMed ] [ Google Scholar ]
- 3. Cullen B.R. How do viruses avoid inhibition by endogenous cellular microRNAs? PLoS Pathog. 2013;9(11):e1003694. doi: 10.1371/journal.ppat.1003694. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 4. Luke G.A., Roulston C., Odon V., de Felipe P., Sukhodub A., Ryan M.D. Lost in translation: The biogenesis of non-LTR retrotransposon proteins. Mob. Genet. Elements. 2013;3(6):e27525. doi: 10.4161/mge.27525. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 5. Katoh I. Impacts of endogenous retroviruses on tumorigenesis, immunity, stem cells, and research safety. Front. Oncol. 2014;4:66. doi: 10.3389/fonc.2014.00066. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 6. Rebollo R., Romanish M.T., Mager D.L. Transposable elements: an abundant and natural source of regulatory sequences for host genes. Annu. Rev. Genet. 2012;46:21–42. doi: 10.1146/annurev-genet-110711-155621. [ DOI ] [ PubMed ] [ Google Scholar ]
- 7. Vitullo P., Sciamanna I., Baiocchi M., Sinibaldi-Vallebona P., Spadafora C. LINE-1 retrotransposon copies are amplified during murine early embryo development. Mol. Reprod. Dev. 2012;79(2):118–127. doi: 10.1002/mrd.22003. [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. Sciamanna I., Gualtieri A., Cossetti C., Osimo E.F., Ferracin M., Macchia G., Aricò E., Prosseda G., Vitullo P., Misteli T., Spadafora C. A tumor-promoting mechanism mediated by retrotransposon-encoded reverse transcriptase is active in human transformed cell lines. Oncotarget. 2013;4(12):2271–2287. doi: 10.18632/oncotarget.1403. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 9. Ohms S., Rangasamy D. Silencing of LINE-1 retrotransposons contributes to variation in small noncoding RNA expression in human cancer cells. Oncotarget. 2014;5(12):4103–4117. doi: 10.18632/oncotarget.1822. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 10. Mallardo M., Poltronieri P., D’Urso O.F. Non-protein coding RNA biomarkers and differential expression in cancers: a review. J. Exp. Clin. Cancer Res. 2008;27:19. doi: 10.1186/1756-9966-27-19. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 11. Ha M., Kim V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014;15(8):509–524. doi: 10.1038/nrm3838. [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Liu J., Rivas F.V., Wohlschlegel J., Yates J.R., III, Parker R., Hannon G.J. A role for the P-body component GW182 in microRNA function. Nat. Cell Biol. 2005;7(12):1261–1266. doi: 10.1038/ncb1333. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 13. Chendrimada T.P., Gregory R.I., Kumaraswamy E., Norman J., Cooch N., Nishikura K., Shiekhattar R. TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nature. 2005;436(7051):740–744. doi: 10.1038/nature03868. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 14. Jin P., Zarnescu D.C., Ceman S., Nakamoto M., Mowrey J., Jongens T.A., Nelson D.L., Moses K., Warren S.T. Biochemical and genetic interaction between the fragile X mental retardation protein and the microRNA pathway. Nat. Neurosci. 2004;7(2):113–117. doi: 10.1038/nn1174. [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Homan P.J., Favorov O.V., Lavender C.A., Kursun O., Ge X., Busan S., Dokholyan N.V., Weeks K.M. Single-molecule correlated chemical probing of RNA. Proc. Natl. Acad. Sci. USA. 2014;111(38):13858–13863. doi: 10.1073/pnas.1407306111. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 16. Siegfried N.A., Busan S., Rice G.M., Nelson J.A., Weeks K.M. RNA motif discovery by SHAPE and mutational profiling (SHAPE-MaP). Nat. Methods. 2014;11(9):959–965. doi: 10.1038/nmeth.3029. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 17. Schroeder S.J. Advances in RNA structure prediction from sequence: new tools for generating hypotheses about viral RNA structure-function relationships. J. Virol. 2009;83(13):6326–6334. doi: 10.1128/JVI.00251-09. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 18. Buenrostro J.D., Araya C.L., Chircus L.M., Layton C.J., Chang H.Y., Snyder M.P., Greenleaf W.J. Quantitative analysis of RNA-protein interactions on a massively parallel array reveals biophysical and evolutionary landscapes. Nat. Biotechnol. 2014;32(6):562–568. doi: 10.1038/nbt.2880. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 19. Bergmann M., Muster T. Mutations in the nonconserved noncoding sequences of the influenza A virus segments affect viral vRNA formation. Virus Res. 1996;44(1):23–31. doi: 10.1016/0168-1702(96)01335-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- 20. Toennessen R., Lauscher A., Rimstad E. Comparative aspects of infectious salmon anemia virus, an orthomyxovirus of fish, to influenza viruses. Indian J. Microbiol. 2009;49(4):308–314. doi: 10.1007/s12088-009-0055-4. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 21. Desselberger U., Racaniello V.R., Zazra J.J., Palese P. The 3′ and 5′-terminal sequences of influenza A, B and C virus RNA segments are highly conserved and show partial inverted complementarity. Gene. 1980;8(3):315–328. doi: 10.1016/0378-1119(80)90007-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. Sandvik T., Rimstad E., Mjaaland S. The viral RNA 3′- and 5′-end structure and mRNA transcription of infectious salmon anaemia virus resemble those of influenza viruses. Arch. Virol. 2000;145(8):1659–1669. doi: 10.1007/s007050070082. [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. Díaz A., García K., Navarrete A., Higuera G., Romero J. Virtual screening of gene expression regulatory sites in non-coding regions of the infectious salmon anemia virus. BMC Res. Notes. 2014;7:477. doi: 10.1186/1756-0500-7-477. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 24. Crescenzo-Chaigne B., Barbezange C., van der Werf S. Non coding extremities of the seven influenza virus type C vRNA segments: effect on transcription and replication by the type C and type A polymerase complexes. Virol. J. 2008;5:132. doi: 10.1186/1743-422X-5-132. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 25. Lee Y.S., Seong B.L. Nucleotides in the panhandle structure of the influenza B virus virion RNA are involved in the specificity between influenza A and B viruses. J. Gen. Virol. 1998;79(Pt 4):673–681. doi: 10.1099/0022-1317-79-4-673. [ DOI ] [ PubMed ] [ Google Scholar ]
- 26. Fodor E., Pritlove D.C., Brownlee G.G. The influenza virus panhandle is involved in the initiation of transcription. J. Virol. 1994;68(6):4092–4096. doi: 10.1128/jvi.68.6.4092-4096.1994. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 27. Brinson R.G., Szakal A.L., Marino J.P. Structural characterization of the viral and cRNA panhandle motifs from the infectious salmon anemia virus. J. Virol. 2011;85(24):13398–13408. doi: 10.1128/JVI.06250-11. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 28. Fassati A., Görlich D., Harrison I., Zaytseva L., Mingot J.M. Nuclear import of HIV-1 intracellular reverse transcription complexes is mediated by importin 7. EMBO J. 2003;22(14):3675–3685. doi: 10.1093/emboj/cdg357. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 29. Kamata M., Nitahara-Kasahara Y., Miyamoto Y., Yoneda Y., Aida Y. Importin-alpha promotes passage through the nuclear pore complex of human immunodeficiency virus type 1 Vpr. J. Virol. 2005;79(6):3557–3564. doi: 10.1128/JVI.79.6.3557-3564.2005. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 30. Zhou H., Xu M., Huang Q., Gates A.T., Zhang X.D., Castle J.C., Stec E., Ferrer M., Strulovici B., Hazuda D.J., Espeseth A.S. Genome-scale RNAi screen for host factors required for HIV replication. Cell Host Microbe. 2008;4(5):495–504. doi: 10.1016/j.chom.2008.10.004. [ DOI ] [ PubMed ] [ Google Scholar ]
- 31. Yeung M.L., Houzet L., Yedavalli V.S., Jeang K.T. A genome-wide short hairpin RNA screening of jurkat T-cells for human proteins contributing to productive HIV-1 replication. J. Biol. Chem. 2009;284(29):19463–19473. doi: 10.1074/jbc.M109.010033. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 32. Houzet L., Jeang K.T. Genome-wide screening using RNA interference to study host factors in viral replication and pathogenesis. Exp. Biol. Med. (Maywood) 2011;236(8):962–967. doi: 10.1258/ebm.2010.010272. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 33. Lever A.M., Jeang K.T. Insights into cellular factors that regulate HIV-1 replication in human cells. Biochemistry. 2011;50(6):920–931. doi: 10.1021/bi101805f. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 34. Kincaid R.P., Burke J.M., Sullivan C.S. RNA virus microRNA that mimics a B-cell oncomiR. Proc. Natl. Acad. Sci. USA. 2012;109(8):3077–3082. doi: 10.1073/pnas.1116107109. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 35. Umbach J.L., Yen H-L., Poon L.L., Cullen B.R. Influenza A virus expresses high levels of an unusual class of small viral leader RNAs in infected cells. MBio. 2010;1(4):e00204–e00210. doi: 10.1128/mBio.00204-10. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 36. Shi J., Sun J., Wang B., Wu M., Zhang J., Duan Z., Wang H., Hu N., Hu Y. Novel microRNA-like viral small regulatory RNAs arising during human hepatitis A virus infection. FASEB J. 2014;28(10):4381–4393. doi: 10.1096/fj.14-253534. [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Weng K.F., Hung C.T., Hsieh P.T., Li M.L., Chen G.W., Kung Y.A., Huang P.N., Kuo R.L., Chen L.L., Lin J.Y., Wang R.Y., Chen S.J., Tang P., Horng J.T., Huang H.I., Wang J.R., Ojcius D.M., Brewer G., Shih S.R. A cytoplasmic RNA virus generates functional viral small RNAs and regulates viral IRES activity in mammalian cells. Nucleic Acids Res. 2014;42(20):12789–12805. doi: 10.1093/nar/gku952. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 38. Kincaid R.P., Sullivan C.S. Virus-encoded microRNAs: an overview and a look to the future. PLoS Pathog. 2012;8(12):e1003018. doi: 10.1371/journal.ppat.1003018. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 39. Omoto S., Fujii Y.R. Regulation of human immunodeficiency virus 1 transcription by nef microRNA. J. Gen. Virol. 2005;86(Pt 3):751–755. doi: 10.1099/vir.0.80449-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 40. Qureshi A., Thakur N., Monga I., Thakur A., Kumar M. VIRmiRNA: a comprehensive resource for experimentally validated viral miRNAs and their targets. Database (Oxford) 2014;2014:bau103. doi: 10.1093/database/bau103. [Oxford]. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 41. Zhang Y., Fan M., Geng G., Liu B., Huang Z., Luo H., Zhou J., Guo X., Cai W., Zhang H. A novel HIV-1-encoded microRNA enhances its viral replication by targeting the TATA box region. Retrovirology. 2014;11:23. doi: 10.1186/1742-4690-11-23. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 42. Klase Z., Kale P., Winograd R., Gupta M.V., Heydarian M., Berro R., McCaffrey T., Kashanchi F. HIV-1 TAR element is processed by Dicer to yield a viral micro-RNA involved in chromatin remodeling of the viral LTR. BMC Mol. Biol. 2007;8:63. doi: 10.1186/1471-2199-8-63. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 43. Ouellet D.L., Plante I., Landry P., Barat C., Janelle M.E., Flamand L., Tremblay M.J., Provost P. Identification of functional microRNAs released through asymmetrical processing of HIV-1 TAR element. Nucleic Acids Res. 2008;36(7):2353–2365. doi: 10.1093/nar/gkn076. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 44. Klase Z., Winograd R., Davis J., Carpio L., Hildreth R., Heydarian M., Fu S., McCaffrey T., Meiri E., Ayash-Rashkovsky M., Gilad S., Bentwich Z., Kashanchi F. HIV-1 TAR miRNA protects against apoptosis by altering cellular gene expression. Retrovirology. 2009;6(1):18. doi: 10.1186/1742-4690-6-18. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 45. Schopman N.C., Willemsen M., Liu Y.P., Bradley T., van Kampen A., Baas F., Berkhout B., Haasnoot J. Deep sequencing of virus-infected cells reveals HIV-encoded small RNAs. Nucleic Acids Res. 2012;40(1):414–427. doi: 10.1093/nar/gkr719. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 46. Clerc I., Laverdure S., Torresilla C., Landry S., Borel S., Vargas A., Arpin-André C., Gay B., Briant L., Gross A., Barbeau B., Mesnard J.M. Polarized expression of the membrane ASP protein derived from HIV-1 antisense transcription in T cells. Retrovirology. 2011;8:74. doi: 10.1186/1742-4690-8-74. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 47. Barbagallo M.S., Birch K.E., Deacon N.J., Mosse J.A. Potential control of human immunodeficiency virus type 1 asp expression by alternative splicing in the upstream untranslated region. DNA Cell Biol. 2012;31(7):1303–1313. doi: 10.1089/dna.2011.1585. [ DOI ] [ PubMed ] [ Google Scholar ]
- 48. Kobayashi-Ishihara M., Yamagishi M., Hara T., Matsuda Y., Takahashi R., Miyake A., Nakano K., Yamochi T., Ishida T., Watanabe T. HIV-1-encoded antisense RNA suppresses viral replication for a prolonged period. Retrovirology. 2012;9:38. doi: 10.1186/1742-4690-9-38. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 49. Swaminathan G., Martin-Garcia J., Navas-Martin S. RNA viruses and microRNAs: challenging discoveries for the 21st century. Physiol. Genomics. 2013;45(22):1035–1048. doi: 10.1152/physiolgenomics.00112.2013. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 50. Lecellier C.H., Dunoyer P., Arar K., Lehmann-Che J., Eyquem S., Himber C., Saïb A., Voinnet O. A cellular microRNA mediates antiviral defense in human cells. Science. 2005;308(5721):557–560. doi: 10.1126/science.1108784. [ DOI ] [ PubMed ] [ Google Scholar ]
- 51. Klase Z., Houzet L., Jeang K.T. MicroRNAs and HIV-1: complex interactions. J. Biol. Chem. 2012;287(49):40884–40890. doi: 10.1074/jbc.R112.415448. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 52. Jopling C. Liver-specific microRNA-122: Biogenesis and function. RNA Biol. 2012;9(2):137–142. doi: 10.4161/rna.18827. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 53. Zhang H.S., Wu T.C., Sang W.W., Ruan Z. MiR-217 is involved in Tat-induced HIV-1 long terminal repeat (LTR) transactivation by down-regulation of SIRT1. Biochim. Biophys. Acta. 2012;1823(5):1017–1023. doi: 10.1016/j.bbamcr.2012.02.014. [ DOI ] [ PubMed ] [ Google Scholar ]
- 54. Sung T.L., Rice A.P. miR-198 inhibits HIV-1 gene expression and replication in monocytes and its mechanism of action appears to involve repression of cyclin T1. PLoS Pathog. 2009;5(1):e1000263. doi: 10.1371/journal.ppat.1000263. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 55. Hariharan M., Scaria V., Pillai B., Brahmachari S.K. Targets for human encoded microRNAs in HIV genes. Biochem. Biophys. Res. Commun. 2005;337(4):1214–1218. doi: 10.1016/j.bbrc.2005.09.183. [ DOI ] [ PubMed ] [ Google Scholar ]
- 56. Nathans R., Chu C.Y., Serquina A.K., Lu C.C., Cao H., Rana T.M. Cellular microRNA and P bodies modulate host-HIV-1 interactions. Mol. Cell. 2009;34(6):696–709. doi: 10.1016/j.molcel.2009.06.003. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 57. Sun G., Li H., Wu X., Covarrubias M., Scherer L., Meinking K., Luk B., Chomchan P., Alluin J., Gombart A.F., Rossi J.J. Interplay between HIV-1 infection and host microRNAs. Nucleic Acids Res. 2012;40(5):2181–2196. doi: 10.1093/nar/gkr961. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 58. Huang J., Wang F., Argyris E., Chen K., Liang Z., Tian H., Huang W., Squires K., Verlinghieri G., Zhang H. Cellular microRNAs contribute to HIV-1 latency in resting primary CD4+ T lymphocytes. Nat. Med. 2007;13(10):1241–1247. doi: 10.1038/nm1639. [ DOI ] [ PubMed ] [ Google Scholar ]
- 59. Wang X., Ye L., Hou W., Zhou Y., Wang Y.J., Metzger D.S., Ho W.Z. Cellular microRNA expression correlates with susceptibility of monocytes/macrophages to HIV-1 infection. Blood. 2009;113(3):671–674. doi: 10.1182/blood-2008-09-175000. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 60. Houzet L., Klase Z., Yeung M.L., Wu A., Le S.Y., Quiñones M., Jeang K.T. The extent of sequence complementarity correlates with the potency of cellular miRNA-mediated restriction of HIV-1. Nucleic Acids Res. 2012;40(22):11684–11696. doi: 10.1093/nar/gks912. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 61. Ma L., Shen C.J., Cohen É.A., Xiong S.D., Wang J.H. miRNA-1236 inhibits HIV-1 infection of monocytes by repressing translation of cellular factor VprBP. PLoS One. 2014;9(6):e99535. doi: 10.1371/journal.pone.0099535. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 62. Hou W., Bonkovsky H.L. Non-coding RNAs in hepatitis C-induced hepatocellular carcinoma: dysregulation and implications for early detection, diagnosis and therapy. World J. Gastroenterol. 2013;19(44):7836–7845. doi: 10.3748/wjg.v19.i44.7836. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 63. Leung A.K., Vyas S., Rood J.E., Bhutkar A., Sharp P.A., Chang P. Poly(ADP-ribose) regulates stress responses and microRNA activity in the cytoplasm. Mol. Cell. 2011;42(4):489–499. doi: 10.1016/j.molcel.2011.04.015. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 64. Lloyd R.E. How do viruses interact with stress-associated RNA granules? PLoS Pathog. 2012;8(6):e1002741. doi: 10.1371/journal.ppat.1002741. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 65. Banfield B.W., Mouland A.J., McCormick C. 1st international symposium on stress-associated RNA granules in human disease and viral infection. Viruses. 2014;6(9):3500–3513. doi: 10.3390/v6093500. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 66. Srivastava S., Swanson S.K., Manel N., Florens L., Washburn M.P., Skowronski J. Lentiviral Vpx accessory factor targets VprBP/DCAF1 substrate adaptor for cullin 4 E3 ubiquitin ligase to enable macrophage infection. PLoS Pathog. 2008;4(5):e1000059. doi: 10.1371/journal.ppat.1000059. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 67. Wang X., Singh S., Jung H.Y., Yang G., Jun S., Sastry K.J., Park J.I. HIV-1 Vpr protein inhibits telomerase activity via the EDD-DDB1-VPRBP E3 ligase complex. J. Biol. Chem. 2013;288(22):15474–15480. doi: 10.1074/jbc.M112.416735. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 68. Nakagawa T., Mondal K., Swanson P.C. VprBP (DCAF1): a promiscuous substrate recognition subunit that incorporates into both RING-family CRL4 and HECT-family EDD/UBR5 E3 ubiquitin ligases. BMC Mol. Biol. 2013;14:22. doi: 10.1186/1471-2199-14-22. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 69. Hakata Y., Miyazawa M., Landau N.R. Interactions with DCAF1 and DDB1 in the CRL4 E3 ubiquitin ligase are required for Vpr-mediated G2 arrest. Virol. J. 2014;11:108. doi: 10.1186/1743-422X-11-108. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 70. Romani B., Cohen E.A. Lentivirus Vpr and Vpx accessory proteins usurp the cullin4-DDB1 (DCAF1) E3 ubiquitin ligase. Curr. Opin. Virol. 2012;2(6):755–763. doi: 10.1016/j.coviro.2012.09.010. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 71. Cohen E.A. From arrest to escape: HIV-1 Vpr cuts a deal. Cell Host Microbe. 2014;15(2):125–127. doi: 10.1016/j.chom.2014.01.012. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 72. Shapshak P. Molecule of the month: HIV-1 protein Vpr and miRNA. Bioinformation. 2012;8(25):1243–1244. doi: 10.6026/97320630081243. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 73. Casey Klockow L., Sharifi H.J., Wen X., Flagg M., Furuya A.K., Nekorchuk M., de Noronha C.M. The HIV-1 protein Vpr targets the endoribonuclease Dicer for proteasomal degradation to boost macrophage infection. Virology. 2013;444(1-2):191–202. doi: 10.1016/j.virol.2013.06.010. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 74. Deng A., Chen C., Ishizaka Y., Chen X., Sun B., Yang R. Human immunodeficiency virus type 1 Vpr increases hepatitis C virus RNA replication in cell culture. Virus Res. 2014;184:93–102. doi: 10.1016/j.virusres.2014.02.017. [ DOI ] [ PubMed ] [ Google Scholar ]
- 75. Okumura A., Alce T., Lubyova B., Ezelle H., Strebel K., Pitha P.M. HIV-1 accessory proteins VPR and Vif modulate antiviral response by targeting IRF-3 for degradation. Virology. 2008;373(1):85–97. doi: 10.1016/j.virol.2007.10.042. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 76. Zhou D., Wang Y., Tokunaga K., Huang F., Sun B., Yang R. The HIV-1 accessory protein Vpr induces the degradation of the anti-HIV-1 agent APOBEC3G through a VprBP-mediated proteasomal pathway. Virus Res. 2015;195:25–34. doi: 10.1016/j.virusres.2014.08.021. [ DOI ] [ PubMed ] [ Google Scholar ]
- 77. Yuan T., Yao W., Huang F., Sun B., Yang R. The human antiviral factor TRIM11 is under the regulation of HIV-1 Vpr. PLoS One. 2014;9(8):e104269. doi: 10.1371/journal.pone.0104269. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 78. Ahn J., Vu T., Novince Z., Guerrero-Santoro J., Rapic-Otrin V., Gronenborn A.M. HIV-1 Vpr loads uracil DNA glycosylase-2 onto DCAF1, a substrate recognition subunit of a cullin 4A-ring E3 ubiquitin ligase for proteasome-dependent degradation. J. Biol. Chem. 2010;285(48):37333–37341. doi: 10.1074/jbc.M110.133181. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 79. Laguette N., Brégnard C., Hue P., Basbous J., Yatim A., Larroque M., Kirchhoff F., Constantinou A., Sobhian B., Benkirane M. Premature activation of the SLX4 complex by Vpr promotes G2/M arrest and escape from innate immune sensing. Cell. 2014;156(1-2):134–145. doi: 10.1016/j.cell.2013.12.011. [ DOI ] [ PubMed ] [ Google Scholar ]
- 80. Zahoor M.A., Xue G., Sato H., Murakami T., Takeshima S.N., Aida Y. HIV-1 Vpr induces interferon-stimulated genes in human monocyte-derived macrophages. PLoS One. 2014;9(8):e106418. doi: 10.1371/journal.pone.0106418. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 81. Collins D.R., Collins K.L. HIV-1 accessory proteins adapt cellular adaptors to facilitate immune evasion. PLoS Pathog. 2014;10(1):e1003851. doi: 10.1371/journal.ppat.1003851. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 82. Foley J.F., Yu C.R., Solow R., Yacobucci M., Peden K.W., Farber J.M. Roles for CXC chemokine ligands 10 and 11 in recruiting CD4+ T cells to HIV-1-infected monocyte-derived macrophages, dendritic cells, and lymph nodes. J. Immunol. 2005;174(8):4892–4900. doi: 10.4049/jimmunol.174.8.4892. [ DOI ] [ PubMed ] [ Google Scholar ]
- 83. Desimmie B.A., Delviks-Frankenberrry K.A., Burdick R.C., Qi D., Izumi T., Pathak V.K. Multiple APOBEC3 restriction factors for HIV-1 and one Vif to rule them all. J. Mol. Biol. 2014;426(6):1220–1245. doi: 10.1016/j.jmb.2013.10.033. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 84. Lu L., Yu F., Du L.Y., Xu W., Jiang S.B. Tactics used by HIV-1 to evade host innate, adaptive, and intrinsic immunities. Chin. Med. J. (Engl.) 2013;126(12):2374–2379. [ PubMed ] [ Google Scholar ]
- 85. Guo F., Liang C. Transmembrane interactions of HIV-1 Vpu and tetherin. Curr. HIV Res. 2012;10(4):292–297. doi: 10.2174/157016212800792450. [ DOI ] [ PubMed ] [ Google Scholar ]
- 86. Schröfelbauer B., Yu Q., Zeitlin S.G., Landau N.R. Human immunodeficiency virus type 1 Vpr induces the degradation of the UNG and SMUG uracil-DNA glycosylases. J. Virol. 2005;79(17):10978–10987. doi: 10.1128/JVI.79.17.10978-10987.2005. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 87. Sze A., Olagnier D., Lin R., van Grevenynghe J., Hiscott J. SAMHD1 host restriction factor: a link with innate immune sensing of retrovirus infection. J. Mol. Biol. 2013;425(24):4981–4994. doi: 10.1016/j.jmb.2013.10.022. [ DOI ] [ PubMed ] [ Google Scholar ]
- 88. Brass A.L., Dykxhoorn D.M., Benita Y., Yan N., Engelman A., Xavier R.J., Lieberman J., Elledge S.J. Identification of host proteins required for HIV infection through a functional genomic screen. Science. 2008;319(5865):921–926. doi: 10.1126/science.1152725. [ DOI ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (205.1 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Drexler M; Institute of Medicine (US). What You Need to Know About Infectious Disease. Washington (DC): National Academies Press (US); 2010.

What You Need to Know About Infectious Disease.
- Hardcopy Version at National Academies Press
I How Infection Works

There is a close connection between microbes and humans. Experts believe about half of all human DNA originated from viruses that infected and embedded their nucleic acid in our ancestors’ egg and sperm cells.
Microbes occupy all of our body surfaces, including the skin, gut, and mucous membranes. In fact, our bodies contain at least 10 times more bacterial cells than human ones, blurring the line between where microbes end and humans begin. Microbes in the human gastrointestinal tract alone comprise at least 10 trillion organisms, representing more than 1,000 species, which are thought to prevent the gut from being colonized by disease-causing organisms. Among their other beneficial roles, microbes synthesize vitamins, break down food into absorbable nutrients, and stimulate our immune systems.
The vast majority of microbes establish themselves as persistent “colonists,” thriving in complex communities within and on our bodies. In many cases, the microbes derive benefits without harming us; in other cases, both host and microbe benefit.
From the moment we are born, microbes begin to colonize our bodies. Each of us has a unique set of microbial communities, which are believed to play an important role in digestion and in protection from disease.
Lactobacillus bacteria, which produce lactic acid to help with digestion.
And though some microbes make us sick and even kill us, in the long run they have a shared interest in our survival. For these tiny invaders, a dead host is a dead end.
The success of microorganisms is due to their remarkable adaptability. Through natural selection, organisms that are genetically better suited to their surroundings have more offspring and transmit their desirable traits to future generations. This process operates far more efficiently in the microbial world than in people. Humans produce a new generation every 20 years or so; bacteria do it every 20 to 30 minutes, and viruses even faster. Because they reproduce so quickly, microorganisms can assemble in enormous numbers with great variety in their communities. If their environment suddenly changes, the community’s genetic variations make it more likely that some will survive. This gives microbes a huge advantage over humans when it comes to adapting for survival.
- Types of Microbes
There are five major categories of infectious agents: Viruses, bacteria, fungi, protozoa, and helminths.
Viruses are tiny, ranging in size from about 20 to 400 nanometers in diameter (see page 9). Billions can fit on the head of a pin. Some are rod shaped; others are round and 20 sided; and yet others have fanciful forms, with multisided “heads” and cylindrical “tails.”
Viruses are simply packets of nucleic acid, either DNA or RNA, surrounded by a protein shell and sometimes fatty materials called lipids. Outside a living cell, a virus is a dormant particle, lacking the raw materials for reproduction. Only when it enters a host cell does it go into action, hijacking the cell’s metabolic machinery to produce copies of itself that may burst out of infected cells or simply bud off a cell membrane. This lack of self-sufficiency means that viruses cannot be cultured in artificial media for scientific research or vaccine development; they can be grown only in living cells, fertilized eggs, tissue cultures, or bacteria.
An electron micrograph of an influenza virus particle, showing details of its structure.
Viruses are responsible for a wide range of diseases, including the common cold, measles, chicken pox, genital herpes, and influenza. Many of the emerging infectious diseases, such as AIDS and SARS, are caused by viruses.
Bacteria are 10 to 100 times larger than viruses and are more self-sufficient. These single-celled organisms, generally visible under a low-powered microscope, come in three shapes: spherical (coccus), rodlike (bacillus), and curved (vibrio, spirillum, or spirochete).
Most bacteria carry a single circular molecule of DNA, which encodes (or programs) the essential genes for reproduction and other cellular functions. Sometimes they carry accessory small rings of DNA, known as plasmids, that encode for specialized functions like antibiotic resistance. Unlike more complex forms of life, bacteria carry only one set of chromosomes instead of two. They reproduce by dividing into two cells, a process called binary fission. Their offspring are identical, essentially clones with the exact same genetic material. When mistakes are made during replication and a mutation occurs, it creates variety within the population that could—under the right circumstances—lead to an enhanced ability to adapt to a changing environment. Bacteria can also acquire new genetic material from other bacteria, viruses, plants, and even yeasts. This ability means they can evolve suddenly and rapidly instead of slowly adapting.
E. coli bacteria directly transferring genetic material via a pilus (the thin strand connecting the two).
Bacteria are ancient organisms. Evidence for them exists in the fossil record from more than 3 billion years ago. They have evolved many different behaviors over a wide range of habitats, learning to adhere to cells, make paralyzing poisons and other toxins, evade or suppress our bodies’ defenses, and resist drugs and the immune system’s antibodies. Bacterial infections are associated with diseases such as strep throat, tuberculosis, staph skin infections, and urinary tract and bloodstream infections.
Other Infectious Agents
The other three major types of infectious agents include fungi (spore-forming organisms that range from bread mold to ringworm to deadly histoplasmosis), protozoa (such as the agents behind malaria and dysentery), and helminths (parasitic worms like those that cause trichinosis, hookworm, and schistosomiasis).
A newly recognized class of infectious agents—the prions, or proteinaceous infectious particles—consist only of protein. Prions are thought to cause variant Creutzfeldt-Jakob disease in humans and “mad cow disease” in cattle. These proteins are abnormally folded and, when they come in contact with similar normal proteins, turn them into prions like themselves, setting off a chain reaction that eventually riddles the brain with holes. Prions evoke no immune response and resist heat, ultraviolet light, radiation, and sterilization, making them difficult to control.
Grand Prismatic Spring, a geothermal hot spring in Yellowstone and home to microbes that have adapted to this extreme environment.
- Encountering Microbes
Microbes have inhabited the earth for billions of years and may be the earliest life forms on the planet. They live in every conceivable ecological niche—soil, water, air, plants, rocks, and animals. They even live in extreme environments, such as hot springs, deep ocean thermal vents, and Antarctic ice. Indeed microbes, by sheer mass, are the earth’s most abundant life form and are highly adaptable to external forces.
New Meeting Places
Any changes that create new intersections between microbes and people pave the way for disease-causing agents to enter our species. One such change that has put us at risk is the global human population explosion—from about 1.6 billion people in 1900 to nearly 7 billion today. Humans have cleared forests for agriculture and suburbanization, leading to closer contact with environments that may harbor novel (or newly introduced) pathogens. Through much of the world’s developing tropical regions, the massive expansion of roads and human settlements has also created transition zones filled with opportunities for contact with potential disease-causing agents.
Human travel and commerce have brought other risks. Almost 2 million passengers, each a potential carrier of infection, travel daily by aircraft to international destinations. International commerce, especially in foodstuffs, adds to the global traffic of disease-causing microbes. Because the transit times of people and goods are often shorter than the incubation periods of infection, carriers of disease can arrive at their destination before the infection they harbor is detectable. International trade and travel are associated with the emergence of such infectious agents as the SARS coronavirus and West Nile virus.

Changes in human demographics and behavior are linked with the emergence of infections such as AIDS and hepatitis C, through sexual activity and intravenous drug use. More broad-scale changes that raise the risk of infectious disease include the breakdown of public health systems, poverty, war, and famine.
Entering the Human Host
Microorganisms capable of causing disease—pathogens—usually enter our bodies through the mouth, eyes, nose, or urogenital openings, or through wounds or bites that breach the skin barrier. Organisms can spread—or be transmitted—by several routes.
Contact: Some diseases spread via direct contact with infected skin, mucous membranes, or body fluids. Diseases transmitted this way include cold sores (herpes simplex virus type 1) and sexually transmitted diseases such as AIDS. Pathogens can also be spread by indirect contact when an infected person touches a surface such as a doorknob, countertop, or faucet handle, leaving behind microbes that are then transferred to another person who touches that surface and then touches his or her eye, mouth, or nose. Droplets spread by sneezes, coughs, or simply talking can transmit disease if they come in contact with mucous membranes of the eye, mouth, or nose of another person. SARS, tuberculosis, and influenza are examples of diseases spread by airborne droplet transmission.
Evidence for why it is important to cover your mouth when you sneeze.
Common vehicles: Contaminated food, water, blood, or other vehicles may spread pathogens. Microorganisms like E. coli and Salmonella enter the digestive system in this manner.
Vectors: Creatures such as fleas, mites, ticks, rats, snails, and dogs—called vectors—can also transmit disease. The most common vector for human infection is the mosquito, which transmits malaria, West Nile virus, and yellow fever.

Airborne transmission: Pathogens can also spread when residue from evaporated droplets or dust particles containing microorganisms are suspended in air for long periods of time. Diseases spread by airborne transmission include measles and hantavirus pulmonary syndrome.
HOW TINY ARE MICROBES?
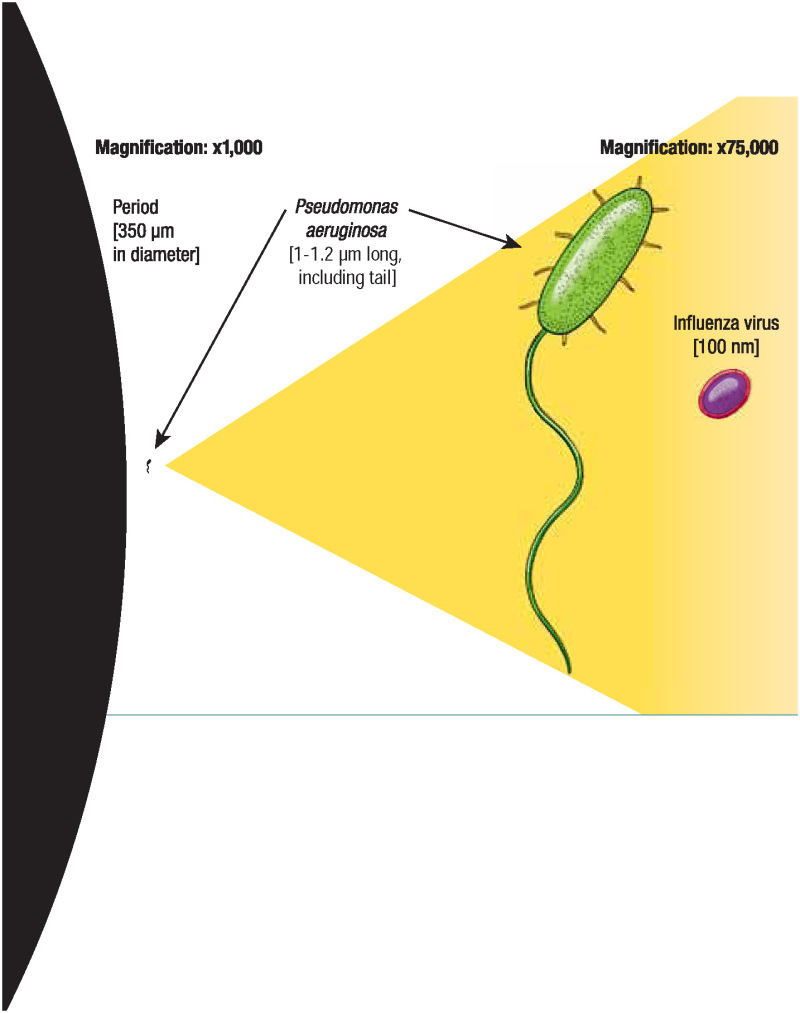
Bacteria and viruses are almost unimaginably small. Bacteria are usually measured in microns (abbreviated “μm,” 1 micron equals 1 one-millionth of a meter), while viruses are measured in the even more miniscule unit of nanometers (abbreviated “nm,” 1 nanometer equals 1 one-billionth of a meter, or 1 one-thousandth of a micron). To give a sense of these measures, consider that the period at the end of this sentence is about 350 microns, or 350,000 nanometers, in diameter. If we magnify the period to one thousand times its actual size (see far left), a nearby Pseudomonas aeruginosa , the bacterium that causes hospital-acquired pneumonia and bloodstream infections, becomes visible. If, in turn, we magnify Pseudomonas 75 more times, or to 75,000 times its actual size, an adjacent influenza virus particle also becomes visible.
- How Pathogens Make Us Sick
Infection does not necessarily lead to disease. Infection occurs when viruses, bacteria, or other microbes enter your body and begin to multiply. Disease, which typically happens in a small proportion of infected people, occurs when the cells in your body are damaged as a result of infection, and signs and symptoms of an illness appear.
In response to infection, your immune system springs into action. White blood cells, antibodies, and other mechanisms go to work to rid your body of the foreign invader. Indeed, many of the symptoms that make a person suffer during an infection—fever, malaise, headache, rash—result from the activities of the immune system trying to eliminate the infection from the body.
Pathogenic microbes challenge the immune system in many ways. Viruses make us sick by killing cells or disrupting cell function. Our bodies often respond with fever (heat inactivates many viruses), the secretion of a chemical called interferon (which blocks viruses from reproducing), or by marshaling the immune system’s antibodies and other cells to target the invader. Many bacteria make us sick the same way, but they also have other strategies at their disposal. Sometimes bacteria multiply so rapidly they crowd out host tissues and disrupt normal function. Sometimes they kill cells and tissues outright. Sometimes they make toxins that can paralyze, destroy cells’ metabolic machinery, or precipitate a massive immune reaction that is itself toxic.
A fever is often part of the immune system’s response to infection.
Other classes of microbes attack the body in different ways:
- Trichinella spiralis , the helminth that causes trichinosis, enters the body encased in cysts residing in undercooked meat. Pepsin and hydrochloric acid in our bodies help free the larvae in the cysts to enter the small intestine, where they molt, mature, and ultimately produce more larvae that pass through the intestine and into the bloodstream. At that point they are free to reach various organs. Those that reach skeletal muscle cells can survive and form new cysts, thus completing their life cycle.
- Histoplasma capsulatum , a fungus that transmits histoplasmosis, grows in soil contaminated with bird or bat droppings. Spores of the fungus emerge from disturbed soil and, once inhaled into the lungs, germinate and transform into budding yeast cells. In its acute phase, the disease causes coughing and flu-like symptoms. Sometimes histoplasmosis affects multiple organ systems and can be fatal unless treated.
- The protozoa that cause malaria, which are members of the genus Plasmodium , have complex life cycles. Sporozoites, a cell type that infects new hosts, develop in the salivary glands of Anopheles mosquitos. They leave the mosquito during a blood meal, enter the host’s liver, and multiply. Cells infected with sporozoites eventually burst, releasing another cell form, merozoites, into the bloodstream. These cells infect red blood cells and then rapidly reproduce, destroying the red blood cell hosts and releasing many new merozoites to do further damage. Most merozoites continue to reproduce in this way, but some differentiate into sexual forms (gametocytes) that are taken up by the female mosquito, thus completing the protozoan life cycle.

These and many other ingenious pathways to causing disease demonstrate pathogens’ rich evolutionary legacy and their continued inventiveness. In the next section, we look more closely at how some of these organisms have learned to thrive—often at humans’ expense.
- Cite this Page Drexler M; Institute of Medicine (US). What You Need to Know About Infectious Disease. Washington (DC): National Academies Press (US); 2010. I, How Infection Works.
- PDF version of this title (1.6M)
In this Page
Recent activity.
- How Infection Works - What You Need to Know About Infectious Disease How Infection Works - What You Need to Know About Infectious Disease
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
Airborne transmission of respiratory viruses
Affiliations.
- 1 Department of Chemistry, National Sun Yat-sen University, Kaohsiung, Taiwan 804, Republic of China. [email protected] [email protected].
- 2 Scripps Institution of Oceanography, University of California San Diego, La Jolla, CA 92037, USA.
- 3 Aerosol Science Research Center, National Sun Yat-sen University, Kaohsiung, Taiwan 804, Republic of China.
- 4 Department of Chemistry, National Sun Yat-sen University, Kaohsiung, Taiwan 804, Republic of China.
- 5 Scripps Institution of Oceanography, University of California San Diego, La Jolla, CA 92037, USA. [email protected] [email protected].
- 6 Department of Biomedical Engineering, Israel Institute of Technology, Haifa 32000, Israel.
- 7 Department of Chemistry and CIRES, University of Colorado, Boulder, CO 80309, USA.
- 8 Department of Microbiology and Molecular Genetics, University of Pittsburgh School of Medicine, Pittsburgh, PA 15219, USA.
- 9 School of Information and Department of Sociology, University of North Carolina, Chapel Hill, NC 27599, USA.
- 10 Department of Civil and Environmental Engineering, Virginia Tech, Blacksburg, VA 24061, USA.
- PMID: 34446582
- PMCID: PMC8721651
- DOI: 10.1126/science.abd9149
The COVID-19 pandemic has revealed critical knowledge gaps in our understanding of and a need to update the traditional view of transmission pathways for respiratory viruses. The long-standing definitions of droplet and airborne transmission do not account for the mechanisms by which virus-laden respiratory droplets and aerosols travel through the air and lead to infection. In this Review, we discuss current evidence regarding the transmission of respiratory viruses by aerosols-how they are generated, transported, and deposited, as well as the factors affecting the relative contributions of droplet-spray deposition versus aerosol inhalation as modes of transmission. Improved understanding of aerosol transmission brought about by studies of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection requires a reevaluation of the major transmission pathways for other respiratory viruses, which will allow better-informed controls to reduce airborne transmission.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Publication types
- Research Support, N.I.H., Extramural
- Research Support, Non-U.S. Gov't
- Research Support, U.S. Gov't, Non-P.H.S.
- Air Microbiology*
- COVID-19 / transmission*
- COVID-19 / virology
- Disease Transmission, Infectious
- Microbial Viability
- Particle Size
- Respiratory System / virology
- Respiratory Tract Infections / transmission*
- Respiratory Tract Infections / virology
- SARS-CoV-2* / isolation & purification
- SARS-CoV-2* / physiology
- Virus Diseases / transmission*
- Virus Diseases / virology
- Virus Physiological Phenomena*
- Viruses / isolation & purification
Grants and funding
- HHSN272201400007C/AI/NIAID NIH HHS/United States
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
Viral infection articles from across Nature Portfolio
Viral infection is the invasion of the body by a small agent known as a virus. Viruses replicate inside host cells and can produce toxins that cause disease. The immune system helps to destroy viruses, but antiviral immune responses can also cause tissue damage and illness.
Latest Research and Reviews
A penta-component mpox mRNA vaccine induces protective immunity in nonhuman primates
Here, the authors report immunogenicity and safety of AR-MPXV5, a penta-component mRNA vaccine, in naive and simian immunodeficiency virus infected nonhuman primates (NHPs), and demonstrate protection in naïve male NHPs after immunization with two doses of AR-MPXV5.
- Cheng-Feng Qin
Electron microscopy images and morphometric data of SARS-CoV-2 variants in ultrathin plastic sections
- Tobias Hoffmann
- Janine Michel
- Michael Laue
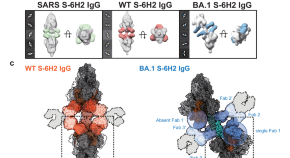
Exploring distinct modes of inter-spike cross-linking for enhanced neutralization by SARS-CoV-2 antibodies
This study demonstrates that a single antibody exhibits distinct binding modes with SARS-CoV-2 WT and Omicron variants, correlating with neutralization loss. The underlying mechanisms offer insights into enhanced neutralization.
- Yuhe R. Yang
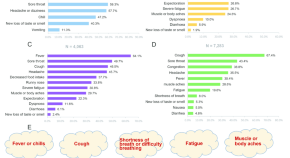
Symptoms can predict COVID-19 pneumonia in patients infected with SARS-CoV-2 Omicron variants
- Qian-Qian Liao
- Ze-Feng Zhu
Compassionate access to virus-specific T cells for adoptive immunotherapy over 15 years
Adoptive T-cell immunotherapy offers promise to patients who are resistant to standard anti-viral strategies. Here the authors describe clinical observations in patients with viral complications treated with adoptive immunotherapy over the last 15 years.
- Michelle A. Neller
- George R. Ambalathingal
- Rajiv Khanna
SARS-CoV-2 human challenge reveals biomarkers that discriminate early and late phases of respiratory viral infections
It’s not always clear whether blood biomarkers are differentially expressed in the time course of viral infections. In this SARS-CoV-2 human challenge study, the authors identify distinct single-gene blood transcriptional biomarkers for early stages of infection or for symptomatic infection.
- Joshua Rosenheim
- Rishi K. Gupta
- Mahdad Noursadeghi
News and Comment

Children exhibit a robust B-cell response to Omicron BA.2 after breakthrough infection with limited influence from the original antigenic sin
- Zhiyang Ling
- Zhangqian Zheng
- Wenhao Zhou
Sialylated IgG restrains lung inflammation
Sialylated IgG protects against severe influenza by inducing the transcriptional repressor REST, which dampens the inflammatory response and preserves lung tissue function.
T H 1 responses in type 2 diabetes
- Stephanie Houston
Innate memory against viruses
- Paula Jauregui

Harnessing symbiotic bacteria for disease control
This Genome Watch explores recent transcriptomic and metatranscriptomic analyses that revealed the key role of secondary endosymbionts in host immunity and disease transmission within their insect or plant hosts.
- Abraham Morales-Cruz
- Leo A. Baumgart
How to prepare for the next inevitable Ebola outbreak: lessons from West Africa
Many lessons have been learned 10 years after the Ebola virus disease outbreak in West Africa, but urgent work is now needed to prevent another outbreak.
- Henry Kyobe Bosa
- Neema Kamara
- Jean Kaseya
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

IMAGES