- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution


Search form
- Advanced search
- Search responses
- Search blogs
- Predicting outcome...
Predicting outcome after traumatic brain injury: practical prognostic models based on large cohort of international patients
- Related content
- Peer review
- MRC CRASH Trial Collaborators
- 1 London School of Hygiene and Tropical Medicine, London WC1B 3DP
- Correspondence to: P A Perel Pablo.perel{at}lshtm.ac.uk
- Accepted 5 December 2007
Objective To develop and validate practical prognostic models for death at 14 days and for death or severe disability six months after traumatic brain injury.
Design Multivariable logistic regression to select variables that were independently associated with two patient outcomes. Two models designed: “basic” model (demographic and clinical variables only) and “CT” model (basic model plus results of computed tomography). The models were subsequently developed for high and low-middle income countries separately.
Setting Medical Research Council (MRC) CRASH Trial.
Subjects 10 008 patients with traumatic brain injury. Models externally validated in a cohort of 8509.
Results The basic model included four predictors: age, Glasgow coma scale, pupil reactivity, and the presence of major extracranial injury. The CT model also included the presence of petechial haemorrhages, obliteration of the third ventricle or basal cisterns, subarachnoid bleeding, midline shift, and non-evacuated haematoma. In the derivation sample the models showed excellent discrimination (C statistic above 0.80). The models showed good calibration graphically. The Hosmer-Lemeshow test also indicated good calibration, except for the CT model in low-middle income countries. External validation for unfavourable outcome at six months in high income countries showed that basic and CT models had good discrimination (C statistic 0.77 for both models) but poorer calibration.
Conclusion Simple prognostic models can be used to obtain valid predictions of relevant outcomes in patients with traumatic brain injury.
Introduction
Traumatic brain injury is a leading cause of death and disability worldwide. Every year, about 1.5 million affected people die and several millions receive emergency treatment. 1 2 Most of the burden (90%) is in low and middle income countries. 3
Clinicians treating patients often make therapeutic decisions based on their assessment of prognosis. According to a 2005 survey, 80% of doctors believed that an accurate assessment of prognosis was important when they made decisions about the use of specific methods of treatment such as hyperventilation, barbiturates, or mannitol. 4 A similar proportion considered that this was important in deciding whether or not to withdraw treatment. Assessment of prognosis was also deemed important for counselling patients and relatives. Only a third of doctors, however, thought that they accurately assessed prognosis. 4
Prognostic models are statistical models that combine data from patients to predict outcome and are likely to be more accurate than simple clinical predictions. 5 The use of computer based prediction of outcome in patients with traumatic brain injury increases the use of certain therapeutic interventions in those predicted to have a good outcome and reduces their use in those predicted to have a poor outcome. 6
Many prognostic models have been reported but none are widely used. A recent systematic review offers possible explanations. 7 Most models were developed on small samples, most were methodologically flawed, and few were validated in external populations. Few were presented in a clinically practical way, nor were they developed in populations from low and middle income countries, where most trauma occurs.
The Medical Research Council (MRC) CRASH (corticosteroid randomisation after significant head injury) trial is the largest clinical trial conducted in patients with traumatic brain injury and presents a unique opportunity to develop a prognostic model. 8 9 The trial prospectively included patients within eight hours of the injury, used standardised definitions of variables, and achieved almost complete follow-up at six months. Furthermore, the large sample size guarantees precise and valid predictions. The high recruitment of patients from low and middle income countries means that models developed with these data are relevant to these settings.
We have developed and validated prognostic models for death at 14 days and death and disability at six months in patients with traumatic brain injury.
Patients —The study cohort was all 10 008 patients enrolled in the trial. Adults with traumatic brain injury, who had a score on the Glasgow coma scale of 14 or less, and who were within eight hours of injury, were eligible for inclusion in the trial.
Outcomes —Death of a patient was recorded on an early outcome form that was completed at hospital discharge, death, or 14 days after randomisation (whichever occurred first). Unfavourable outcome (death or severe disability) at six months was defined with the Glasgow outcome scale (see box). The scale comprises five categories: death, vegetative state, severe disability, moderate disability, and good recovery. For the purpose of this analysis, we dichotomised outcomes into favourable (moderate disability or good recovery) and unfavourable (dead, vegetative state, or severe disability). 10
Category and definition on Glasgow outcome scale
Good recovery: able to return to work or school
Moderate disability: able to live independently; unable to return to work or school
Severe disability: able to follow commands/unable to live independently
Persistent vegetative state: unable to interact with environment; unresponsive
Prognostic variables —For the prognostic model we considered age, sex, cause of injury, time from injury to randomisation, Glasgow coma score at randomisation, pupil reactivity, results of computed tomography, whether the patient had sustained a major extracranial injury, and level of income in country (high or low-middle income countries, as defined by the World Bank) (see table A on bmj.com). 11 We adjusted analyses for treatment within the trial as this was related to outcome, and we did not find interaction between treatment and the potential predictors. 8 9
Analysis —Most of the variables collected in the CRASH trial have been previously associated with prognosis in traumatic brain injury, so we included all of them in a first multivariable logistic regression analysis. 12 We excluded variables that were not significant at 5% level. We quantified each variable’s predictive contribution by its z score (the model coefficient divided by its standard error). We explored linearity between age and mortality at 14 days and Glasgow coma score and mortality at 14 days. Interactions between country income level and all the other predictors were evaluated with a likelihood ratio test. Because there were few data missing, we performed a complete case analysis.
Prognostic models —We developed different models for each of the two outcomes: a basic model, which included only clinical and demographic variables, and a CT model, which also included results of computed tomography.
Performance of the model —We assessed performance of the models in terms of calibration and discrimination. Calibration was assessed graphically and with the Hosmer-Lemeshow test. Discrimination was assessed with the C statistic (an equivalent concept to area under the receiver operator characteristic curve). 13
Internal validation —The internal validity of the final model was assessed by the bootstrap re-sampling technique. Regression models were estimated in 100 models. For each of 100 bootstrap samples we refitted and tested the model on the original sample to obtain an estimate of predictive accuracy corrected for bias. This showed no overoptimism in any of the final model’s predictive C statistics.
External validation —A good prognostic model should be generalisable to populations different to those in which it was derived. 14 We externally validated the models in an external cohort of 8509 patients with moderate and severe traumatic brain injury from 11 studies conducted in high income countries (the IMPACT (international mission for prognosis and clinical trial) dataset). 15
Score development —We developed a clinical score based on regression coefficients. A web based version of the model was developed to be accessible to clinicians internationally.
General characteristics
Table 1 shows the characteristics of the patients. ⇓ More of the patients were men (81%) and more came from low-middle income countries (75%). More than half (58%) of participants were included within three hours of injury. Road traffic crashes were the most common cause of injury (65%) and 79% of the participants underwent computed tomography. A total of 1948 patients (19%) died in the first two weeks, 2323 patients (24%) were dead at six months, and 3556 patients (37%) were dead or severely dependent at six months.
General characteristics of study population
- View inline
The relation between age and the log odds of death within 14 days showed no association until the age of 40 and a linear increase afterwards. The relation between Glasgow coma score and mortality at 14 days was reasonably linear and we therefore included the coma score as a continuous variable (figs 1 and 2) ⇓ ⇓ . The relation with unfavourable outcome at six months showed similar patterns.
Fig 1 Relation between age and mortality at 14 days
- Download figure
- Open in new tab
- Download powerpoint
Fig 2 Relation between Glasgow coma scale and mortality at 14 days
Low-middle v high income countries
In comparison with patients from high income countries, those from low-middle income countries were younger, more likely to be male, were recruited later, had less severe traumatic brain injury (as defined by Glasgow coma score and pupil reactivity), and more often had abnormal results on computed tomography. Road traffic crashes were a more common cause of traumatic brain injury. Although patients from low-middle income countries experienced higher mortality at 14 days (odds ratio 1.94, 95% confidence interval 1.64 to 2.30), there was no significant difference in unfavourable outcome at six months.
There were significant interactions between the country’s income level and several predictors and so we developed two models, one for low-middle income countries and another for high income countries. Older age was a stronger predictor of 14 day mortality in high income countries (interaction P<0.001), and lower Glasgow coma score was a stronger predictor in low-middle income countries (interaction P=0.003). Obliteration of the third ventricle and a non-evacuated haematoma were both associated with a higher risk in high income countries (interaction P<0.001 and P=0.03, respectively).
Multivariable predictive models
We developed eight models altogether: basic and CT models for predicting two outcomes in two settings (low-middle and high income countries).
Basic models —We included four predictors in the basic model: age, Glasgow coma score, pupil reactivity, and the presence of major extracranial injury (table 2). ⇓ Glasgow coma score was the strongest predictor of outcome in low-middle income countries and age was the strongest predictor in high income countries, while the absence of pupil reactivity was the third strongest predictor in both regions.
Multivariable basic predictive models (excluding data from computed tomography*). Figures are odds ratios (95% confidence intervals) with z scores
CT models —The following characteristics on computed tomography were strongly associated with the outcomes in addition to the predictors included in the basic models: presence of petechial haemorrhages, obliteration of the third ventricle or basal cisterns, subarachnoid bleeding, midline shift, and non-evacuated haematoma (table 3). ⇓ Obliteration of the third ventricle and midline shift were the strongest predictors of mortality at 14 days, and non-evacuated haematoma was the strongest predictor of unfavourable outcome at six months.
Multivariable predictive models with computed tomography*. Figures are odds ratios (95% confidence intervals) with z scores
Performance of models —All models showed excellent discrimination, with C statistics over 0.80 (tables 2 and 3). ⇑ ⇑ Calibration in all models was adequate and six out of the eight models had good calibration when evaluated with the Hosmer-Lemeshow test (figs 3 and 4) ⇓ ⇓ .
Fig 3 Calibration of basic models using expected and observed probabilities of mortality at 14 days (top) and death or severe disability at six months (bottom) in patient with traumatic brain injury according to income level of country. P value is for Hosmer-Lemeshow test
Fig 4 Calibration of computed tomography models using expected and observed probabilities of mortality at 14 days (top) and death or severe disability at six months (bottom) in patient with traumatic brain injury according to income level of country. P value is for Hosmer-Lemeshow test
Clinical score —Individual scores and their respective probability of outcome can be obtained from our web based calculator ( www.crash2.lshtm.ac.uk/ ). By entering the values of the predictors, we can obtain the expected risk of death at 14 days and of death or severe disability at six months. Figure 5 shows a sample screenshot of the predictions for a 26 year old patient from a low and middle income country (Argentina), with a Glasgow coma score of 11, one pupil reactive, and absence of a major extra cranial injury. ⇓ According to the basic model this patient has a probability of death at 14 days of 10% and a 23.9% risk of death or severe disability at six months. A good agreement is evident between observed and predicted outcome by the web calculator (figs 3 and 4). ⇑ ⇑
Fig 5 Screenshot of web based calculator available at www.crash2.lshtm.ac.uk/ . If CT scan available box is ticked, calculator displays additional CT variables
External validation —Because an external cohort of patients from low-middle income countries was not available, we validated the models in patients from high income countries only. The IMPACT dataset used for the validation did not include mortality at 14 days and so we could validate only models for unfavourable outcome at six months. We validated the basic model with the variables age, Glasgow coma score, and pupil reactivity. We did not include the variable “major extracranial injury” as it was not available in the validation sample. For the CT models, we added obliteration of the third ventricle or basal cisterns, subarachnoid bleeding, midline shift, and non-evacuated haematoma to the basic model. Similarly, we excluded the variable “petechial haemorrhages” as this was not available in the validation sample. For the validation process we first ran these models in the CRASH trial cohort and we then applied the corresponding coefficients in the validation sample. Although discrimination was, as expected, lower than in the original data, it was still quite good for both the basic and CT models (C statistic 0.77 for both models). The calibration was excellent for the CT model but poorer for the basic model (figs 6 and 7). ⇓ ⇓
Fig 6 External validation of basic model for death or severe disability at six months in IMPACT database
Fig 7 External validation of CT model for death or severe disability at six months in IMPACT database
We have developed web based prognostic models for predicting two clinically relevant outcomes in patients with traumatic brain injury using variables that are available at the bedside. The models have excellent discrimination and good fit with both internal and external validation. We have reported on differences in outcomes and on the strength of predictors of outcomes, according to whether patients are from high or low-middle income countries.
Older age, low Glasgow coma score, absent pupil reactivity, and the presence of major extracranial injury predict poor prognosis. All of these variables have been previously identified as prognostic factors for poor outcome in traumatic brain injury. 12 Glasgow coma score showed a clear linear relation with mortality. Our finding that mortality in patients with Glasgow coma score of 3 was lower than in patients with a score of 4 may be because scores of sedated patients are reported as 3. Increasing age was associated with worse outcomes but this association was apparent only after age 40. A similar threshold has been reported elsewhere. 16 17 Plausible explanations for this include extracranial comorbidities, changes in brain plasticity, or differences in clinical management associated with increasing age. The presence of “obliteration of third ventricle or basal cisterns” on computed tomography was associated with the worst prognosis at 14 days. This is supported by recent findings that absence of basal cisterns is the strongest predictor of six month mortality. 18 We also found—as previously reported—the independent prognostic value of traumatic subarachnoid haemorrhage. 19
Patients from low-middle income countries had worse early prognosis than those from high income countries. Regional differences in outcome after traumatic brain injury have previously been reported between Europe and North America, but the difference in mortality between low-middle and high income countries has not been explored. 20
The strength of association between some predictors and outcomes differed by region. A low Glasgow coma score had an even worse prognosis in patients from low-middle income countries compared with patients from high income countries. This might relate to quality of care or it could be that low Glasgow coma score in high income countries is associated with greater use of sedation, rather than to severity of traumatic brain injury. Increasing age had a worse prognosis in high income countries compared with low-middle income countries. This is because of even lower risks at younger ages in high income countries, while both have similar risks at older ages. Regarding computed tomography, some abnormal findings were stronger predictors in high income countries. This could be because of better technology and therefore more accurate diagnosis with computed tomography.
A systematic review identified over 100 prognostic models for patients with traumatic brain injury, but methodological quality was adequate in only a few. 7 As with our models, two of the more methodologically robust models showed similarly good discrimination but worse calibration. 17 21 They too included Glasgow coma score, age, pupil reactivity, and results of computed tomography as predictors, but, unlike our models, they did not include the presence of major extracranial injury, and none of them included patients from low-middle income countries.
Strengths and weakness of the study
Our study’s strengths are the use of a well described cohort of patients, prospective and standardised collection of data on prognostic factors, low loss to follow-up, and the use of a validated outcome measure at a fixed time after the injury. All of these factors provide reassurance about the internal validity of our models. The large sample size in relation to the number of prognostic variables examined is another particular strength. In relation to its external validity, only a few prognostic models have been developed from patients in low-middle income countries, and to the best of our knowledge the models we developed are the first with a large sample size and adequate methods. 7 The external validation confirmed the discriminatory ability of the models in patients from high income countries and showed good calibration for the computed tomography model. Unlike most published prognostic models, we included the complete spectrum of patients with traumatic brain injury, ranging from mild to severe. Finally, the data required to make predictions with the model are easily available to clinicians, and we have developed a web based risk calculator.
There are some limitations. The data from which the models were developed come from a clinical trial and this could therefore limit external validity. For example, patients were recruited within eight hours of injury and we cannot estimate the accuracy of the models for patients evaluated beyond this time. Nevertheless, the CRASH trial was a pragmatic trial that did not require any additional tests and therefore included a diversity of “real life” patients. Another limitation was that for the validation we were forced to exclude the variables major extracranial injury and petechial haemorrhages because they were not available in the IMPACT sample. Neither of these variables, however, was among the stronger predictors. The external validation showed good discriminatory ability, but this was somewhat lower than in the original data. This may be explained by a more homogeneous selected case mix in these other trials, which included only patients with moderate and severe Glasgow coma score.
Implications of the study
Most of the burden of traumatic brain injury is in low-middle income countries, where case fatality is higher and resources are limited. We found that several predictors differed in their strength of association with outcome according to income level of country, suggesting that it may be inappropriate to extrapolate from models for high income countries to poorer settings. We have developed a methodologically valid, simple, and accurate model that may help decisions about health care for individual patients. It is important to emphasise, however, that while prognostic models may complement clinical decision making they cannot and should not replace clinical judgment. This is particularly important in the context of judgments about the withdrawal of care or clinical triage. These prognostic models can also help in the design and analysis of clinical trials, through prognostic stratification, and can be used in clinical audit by allowing adjustment for case mix. 22
Future research
The differences found between the prognostic models for low-middle and high income countries are important. Although most of the burden of trauma occurs in low-middle income countries, most research takes place in high income countries. 3 A recent systematic review found that few prognostic models for traumatic brain injury were developed in low-middle income countries. 7 More research is therefore needed to obtain reliable data from these settings. An improved understanding of the differences between these regions might also clarify the mechanisms of predictors that are not immediately obvious when we analyse a homogeneous population. Because our models were developed with data from a clinical trial, and validated only in patients from high income countries, further prospective validation in independent cohorts is needed to strengthen the generalisability of the models. Future research could also evaluate different ways, or formats, for presenting the models to physicians; their use in clinical practice; and whether ultimately they have any impact on the management and outcomes of patients with traumatic brain injury.
What is already known on this topic
Traumatic brain injury is a leading cause of death and disability worldwide with most cases occurring in low-middle income countries
Prognostic models may improve predictions of outcome and help in clinical research
Many prognostic models have been published but methodological quality is generally poor, sample sizes small, and only a few models have included patients from low-middle income countries
What this study adds
In a basic model prognostic indicators included age, Glasgow coma scale, pupil reactivity, and the presence of major extracranial injury
In a CT model additional indicators were the presence of petechial haemorrhages, obliteration of the third ventricle or basal cisterns, subarachnoid bleeding, mid-line shift, and non-evacuated haematoma
The strength of predictors of outcomes varies according to whether patients are from high or low-middle income countries
These prognostic models, that include simple variables, are available on the internet ( www.crash2.lshtm.ac.uk/ )
CRASH trial collaborators by country (number of patients randomised)
Albania (41 patients): Fatos Olldashi (national coordinator), Itan Muzha (Central Military University Hospital National Trauma Centre, 35); Nikolin Filipi ( University Hospital “Mother Teresa” Tirana, 6).
Argentina (359 patients): Roberto Lede, Pablo Copertari, Carolina Traverso, Alejandro Copertari (IAMBE—national and regional coordinators for southern Latin America); Enrique Alfredo Vergara, Carolina Montenegro, Roberto Ruiz de Huidobro, Pantaleón Saladino (Hospital San Bernardo, 106); Karina Surt, José Cialzeta, Silvio Lazzeri (Hospital Escuela Jose de San Martin, 52); Gustavo Piñero, Fabiana Ciccioli (Hospital Municipal “Dr Leonidas Lucero,” 37); Walter Videtta, María Fernanda Barboza (Hospital Dr Ramón Carrillo, 35); Silvana Svampa, Victor Sciuto (Hospital Castro Rendon, 28); Gustavo Domeniconi, Marcelo Bustamante (Hospital Zonal General De Agudos “Heroes de Malvinas,” 27); Maximiliano Waschbusch (Policlinico Sofia T de Santamarina. 20); María Paula Gullo (Hospital Municipal Dr Hector J D’Agnillo, 17); Daniel Alberto Drago (Hospital Nacional Profesor Alejandro Posadas, 11); Juan Carlos Arjona Linares (Hospital Español de Mendoza, 10); Luis Camputaro (Hospital Italiano, 10); Gustavo Tróccoli (Hospital “Dr José Penna,” 5); Hernán Galimberti (Hospital Aleman, 1).
Australia (13 patients): Mandy Tallott (Gold Coast Hospital, 13).
Austria (21 patients): Christian Eybner, Walter Buchinger (Waldviertelklinikum Standort Horn, 17); Sylvia Fitzal (Wilhelminenspital der Stadt Wien, 4).
Belgium (403 patients): Guy Mazairac (national coordinator), Véronique Oleffe, Thierry Grollinger, Philippe Delvaux, Laurent Carlier (Centre Hospitalier Regional de Namur, 356); Veronique Braet (AZ Klina Hospital, 34); Jean-Marie Jacques (Hospital of Jolimont, 11); Danielle de Knoop (Clinique Saint-Luc, 2).
Brazil (119 patients): Luiz Nasi (national coordinator), Humberto Kukhuyn Choi, Mara Schmitt (Hospital de Pronto Socorro de Porto Alegre, 113); André Gentil (Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, 5); Flavio Nacul (Clínica São Vicente, 1).
Chile (3 patients): Pedro Bedoya Barrios (Hospital Regional Copiapo, 3).
China (87 patients): Chen Xinkang, Lin Shao Hua, Huang Han Tian (Zhongshan City People’s Hospital, 79); Cai Xiaodong (Sheng Zheng Second People’s Hospital, 8).
Colombia (832 patients): Wilson Gualteros, Alvaro Ardila Otero (Hospital Universitario San Jorge, 216); Miguel Arango (national coordinator, regional coordinator for northern Latin America and Caribbean), Juan Ciro, Hector Jaramillo (Gloria GarciaClinica Las Americas, 199); Ignacio Gonzalez, Carolina Gomez (Hospital General de Medellin, 119); Arturo Arias, Marco Fonseca, Carlos Mora (Hospital Erasmo Meoz, 90); Edgar Giovanni Luna Cabrera, José Luis Betancurth, Porfirio Muñoz (Hospital Departamental de Nariño, 51); Jesus Alberto Quiñónez, Maria Esther Gonzalez Castillo (Hospital San Andres, 37); Orlando Lopez (Hospital Federico Lleras, 31); Rafael Perez Yepes, Diana Leon Cuellar, Gerson Paez (Hospital El Tunal, 24); Hernán Delgado Chaves, Pablo Emilio Ordoñez (Hospital Civil de Ipiales, 21); Ricardo Plata, Martha Pineda (Hospital Universitario del Valle, 15); Libardo Enrique Pulido (Hospital Regional de Duitama, 12); John Sergio Velez Jaramillo (Hospital Timothy Britton, 12); Carlos Rebolledo (Organización Clinica General del Norte, 5).
Costa Rica (20 patients): Oscar Palma (Hospital México, 20).
Cuba (404 patients): Caridad Soler (national coordinator); Irene Pastrana, Raul Falero (Hospital Abel Santamaria Cuadrado, 77); Mario Domínguez Perera, Agustín Arocha García, Raydel Oliva (Hospital Universitario “Arnaldo Milián Castro,” 55); Hubiel López Delgado (Hospital Provincial Docente “Manuel Ascunce Domenech,” 43); Aida Madrazo Carnero, Boris Leyva López (Hospital VI Lenin, 42); Angel Lacerda Gallardo, Amarilys Ortega Morales (Hospital General de Morón, 40); Humberto Lezcano (Hospital General Universitario “Carlos Manuel de Cèspedes,” 38); Marcos Iraola Ferrer (Hospital Universitario “Dr Gustavo Aldereguia Lima,” 37); Irene Zamalea Bess, Gladys Rivas Canino (Hospital Miguel Enriquez, 36); Ernesto Miguel Piferrer Ruiz (Hospital Clínico-Quirúrgico Docente “Saturnino Lora,” 32); Orlando Garcia Cruz (Centro de Investigaciones Médico-Quirúrgicas, 4).
Czech Republic (961 patients): Petr Svoboda (national coordinator), Ilona Kantorová, Jiřĺ Ochmann, Peter Scheer, Ladislav Kozumplík, Jitka Maršová (Research Institute for Special Surgery and Trauma, 852); Karel Edelmann (Masaryk Hospital, 41); Ivan Chytra, Roman Bosman (Charles University Hospital, Plzen, 35); Hana Andrejsová (University Hospital Hradec Kralove, 15); Jan Pachl (Hospital Kralovske Vinohgrady, 9); Jan Bürger (Hospital Pribram, 7); Filip Kramar (Univerzity Karlovy Neurochirurgicka Klinika, 2).
Ecuador (258 patients): Mario Izurieta Ulloa (national coordinator), Luis Gonzalez, Alberto Daccach, Antonio Ortega, Stenio Cevallos (Hospital Luis Vernaza, 202); Boris Zurita Cueva (Hospital de la Policia Guayaquil, 16); Marcelo Ochoa (Hospital Jose Carrasco Arteaga, 11); Jaime Velásquez Tapia (Hospital Naval, 11); Jimmy Hurtado (Clínica Central, 8); Miguel Chung Sang Wong (Hospital Militar de Guayaquil, 5); Roberto Santos (Hospital Regional del IESS “Dr Teodoro Maldonado Carbo,” 5).
Egypt (775 patients): Hussein Khamis (national coordinator), Abdul Hamid Abaza, Abdalla Fekry, Salah El Kordy, Tarek Shawky (Mataria Teaching Hospital, 364); Hesham El-Sayed (national coordinator), Nabil Khalil, Nader Negm, Salem Fisal (Suez Canal University, 180); Mamdouh Alamin, Hany Shokry (Aswan Teaching Hospital, 160); Ahmed Yahia Elhusseny, Atif Radwan, Magdi Rashid (Zagazig University Hospital, 71).
Georgia (56 patients): Tamar Gogichaisvili (national coordinator), George Ingorokva, Nikoloz Gongadze (Neurosurgery Department of Tbilisi State Medical University, 55); Alexander Otarashvili (Tbilisi 4th Hospital, 1).
Germany (27 patients): Waltraud Kleist (Ernst Moritz Arndt University, 14); Mathias Kalkum (Kreiskrankenhaus Tirschenreuth, 8); Peter Ulrich (Klinikum Offenbach, 5).
Ghana (7 patients): Nii Andrews (Narh-Bita Hospital, 7).
Greece (20 patients): George Nakos (University Hospital of Ioannina, 8); Antonios Karavelis (University General Hospital of Larissa, 5); George Archontakis (Chania General Hospital “St George,” 4); Pavlos Myrianthefs (KAT Hospital of Athens, 3).
India (973 patients): Yadram Yadav, Sharda Yadav, R Khatri, Arvind Baghel (NSCB Medical College, 177); Mazhar Husain (national coordinator for north India), Deepak Jha (King George Medical College, 105); Wu Hoong Chhang, Manohar Dhandhania, Choden Fonning (North Bengal Neuro Research Centre, 65); S N Iyengar, Sanjay Gupta (G R Medical College, 51); R R Ravi, K S Bopiah, Ajay Herur (Medical Trust Hospital Kochi, 51); N K Venkataramana (national coordinator for south India), A Satish (Manipal Hospital, 50); K Bhavadasan, Raymond Morris, Ramesh S (Medical College Hospital Trivandrum, 50); A Satish (Abhaya Hospital, 42); Yashbir Dewan, Yashpal Singh (Christian Medical College, 36); Rajesh Bhagchandani, Sanjana Bhagchandani (Apex Hospital Bhopal, 32); Vijaya Ushanath Sethurayar (Meenakshi Mission Hospital and Research Centre, 32); Sojan Ipe, G Sreekumar (MOSC Medical College Hospital, 32); Manas Panigrahi, Agasti Reddy (Nizam’s Institute of Medical Sciences, 28); Varinder Khosla, Sunil Gupta (Postgraduate Institute of Medical Education and Research, 28); Haroon Pillay, Nisha Thomas (Baby Memorial Hospital, 25); Krishnamurthy Sridhar, Bobby Jose (V H S Hospital, 22); Nadakkavvkakan Kurian (Jubilee Mission Hospital, 20); Shanti Praharaj, Shibu Pillai (National Institute of Mental Health and Neurosciences, 17); Ramana (Care Hospital (16); Sanjay Gupta, Smita Gupta (Sri Sai Hospital, 16); Dilip Kiyawat (Hirabi Cowasji Jehangir Medical Research Institute, 15); Kishor Maheshwari (Maheshwari Orthopaedic Hospital, 13); Dilip Panikar (Amrita Institute of Medical Sciences, 11); Jayant Chawla (Hartej Maternity and Nursing Home, 7); Satyanarayana Shenoy, Annaswamy Raja (Kasturba Medical College and Hospital, 7); Yeshomati Rupayana (Choitram Hospital and Research Centre, 6); Suryanarayan Reddy (Gowri Gopal Superspeciality Hospital, 6); Nelanuthala Mohan (Apex Hospital Visakhapatnam, 3); Shailesh Kelkar (Central India Institute of Medical Sciences, 3); Yadram Yadav (Marble City Hospital and Research Centre, 3); Jayant Chawla (Government Medical College Amritsar, 1); Mukesh Johri (Johri Hospital, 1); Yadram Yadav (National Hospital Jabalpur, 1).
Indonesia (238 patients): Nyoman Golden (national coordinator), Sri Maliawan (Sanglah General Hospital, 222); Achmad Fauzi, Umar Farouk (Sidoarjo General Hospital, 14).
Iran (233 patients): Esmaeel Fakharian, Amir Aramesh (Naghavi University Hospital, 110); Maasoumeh Eghtedari, Farhad Ahmadzadeh, Alireza Gholami (Fatemeh Zahra Hospital, 85); Maasoumeh Eghtedari, Farhad Ahmadzadeh (Social Security Hospital, 38).
Ireland (113 patients): Patrick Plunkett, Catherine Redican, Geraldine McMahon (St James’s Hospital, 113).
Italy (9 patients): Maria Giuseppina Annetta (Università Cattolica del Sacro Cuore, 4); Homère Mouchaty (Università di Firenze, 3); Eros Bruzzone (Ospedale San Martino, 2).
Ivory Coast (3 patients): Béatrice Harding (CHU de Cocody, 3).
Kenya (2 patients): Mahmood Qureshi (Aga Khan Hospital, 2).
Malaysia (176 patients): Zamzuri Idris, Jafri Abdullah NC, Ghazaime Ghazali, Abdul Rahman Izaini Ghani (Hospital University Science Malaysia, 162); Fadzli Cheah (Ipoh Specialist Hospital, 14).
Mexico (17 patients): Alfredo Cabrera (national coordinator); José Luis Mejía González (Hospital General Regional No 1, 12); José Luis Mejía González (Hospital General de Queretaro, 4); Jorge Loría-Castellanos (Hospital General Regional No 25, 1).
New Zealand (43 patients): Suzanne Jackson, Robyn Hutchinson (Dunedin Hospital, 43).
Nigeria (180 patients): Edward Komolafe (national coordinator), Augustine Adeolu, Morenikeji Komolafe (Obafemi Awolowo University Teaching Hospitals, 77); Olusanya Adeyemi-Doro, Femi Bankole (Lagos University Teaching Hospital, 43); Bello Shehu, Victoria Danlami (Usmanu Danfodiyo University Teaching Hospital, 36); Olugbenga Odebode (University of Ilorin Teaching Hospital, 15); Kehinde Oluwadiya (Lautech Teaching Hospital, 7); Ahmed Sanni (Lagos State University Teaching Hospital, 1); Herb Giebel (Seventh Day Adventist Hospital, 1); Sushil Kumar (St Stephen’s Hospital, 1).
Pakistan (17 patients): Rashid Jooma (Jinnah Postgraduate Medical Centre, 17).
Panama (7 patients): Jose Edmundo Mezquita (Complejo Hospitalario M A Guerrero, 7).
Paraguay (10 patients): Carlos Ortiz Ovelar (Instituto de Prevision Social, 10).
Peru (8 patients): Marco Gonzales Portillo (Hospital Nacional “Dos de Mayo,” 6); Diana Rodriguez (national coordinator) (Hospital Nacional Arzobispo Loayza, 2).
Romania (319 patients): Laura Balica (national coordinator), Bogdan Oprita, Mircea Sklerniacof, Luiza Steflea, Laura Bandut (Spitalul Clinic de Urgenötùa Bucuresö ti, 282); Adam Danil, Remus Iliescu (Sfantum Pantelimon Hospital, 28); Jean Ciurea (Prof Dr D Bagdasar Clinical Emergency Hospital, 9).
Saudi Arabia (32 patients): Abdelazeem El-Dawlatly, Sherif Alwatidy (King Khalid University Hospital, 24); Walid Al-Yafi, Megahid El-Dawlatly (King Khalid National Guard Hospital, 8).
Serbia (23 patients): Ranka Krunic-Protic, Vesna Janosevic (Klinicki Centar Srbije, 23).
Singapore (23 patients): James Tan (national coordinator) (National Neuroscience Institute, 21); Charles Seah (Changi General Hospital, 2).
Slovakia (179 patients): Štefan Trenkler (national coordinator), Matuš Humenansky, Tatiana Stajančová (Reiman Hospital, 71); Ivan Schwendt, Anton Laincz (NsP Poprad, 39); Zeman Julius, Stano Maros (Nemocnica Bojnice, 25); Jozef Firment (FNsp Kosice, 12); Maria Cifraničova (NsP Trebisov, 11); Beata Sániová (Faculty Hospital in Martin, 10); Karol Kalig (NsP Ruzinov, 4); Soňa Medekova (NsP Nové Zámky, 3); Radovan Wiszt (NsP Liptovsky Mikulas, 2); NsP F D Roosevelt, 1); Ivan Mačuga (NsP Zilina, 1).
South Africa (366 patients): Bennie Hartzenberg (national coordinator), Grant du Plessis, Zelda Houlie (Tygerberg Academic Hospital, 307); Narendra Nathoo, Sipho Khumalo (Wentworth Hospital, 57); Ralph Tracey (Curamed Kloof Hospital, 1).
Spain (259 patients): Angeles Muñoz-Sánchez (national coordinator), Francisco Murillo-Cabezas NC, Juan Flores-Cordero, Dolores Rincón-Ferrari (Hospital Universitario Virgen del Rocio, 133); Martin Rubi, Lopez Caler (Hospital Torrecárdenas, 37); Maite Misis del Campo, Luisa Bordejé Laguna (Hospital Universitario Germans Trias i Pujol, 32); Juan Manuel Nava (Hospital Mútua de Terrassa, 20); Miguel Arruego Minguillón (Hospital Universitario de Girona Dr Josep Trueta, 12); Alfonso Muñoz Lopez (Hospital Carlos Haya, 10); Luis Ramos-Gómez (Hospital General de La Palma, 6); Victoria de la Torre-Prados (Hospital Universitario Virgen de la Victoria, 5); Romero Pellejero (Hospital General Yagüe, 4).
Sri Lanka (132 patients): Véronique Laloë (national coordinator), Bernhard Mandrella, Suganthan (Batticaloa General Hospital-Médecins Sans Frontières, 84); Sunil Perera (National Hospital of Sri Lanka, 39); Véronique Laloë, Kanapathipillai Mahendran (Point-Pedro Base Hospital, 9).
Switzerland (160 patients): Reto Stocker (national coordinator), Silke Ludwig (national coordinator) (University Hospital of Zurich, 133); Heinz Zimmermann (University Hospital Bern, 15); Urs Denzler (Kantonsspital Schaffhausen, 12).
Thailand (579 patients): Surakrant Yutthakasemsunt (national coordinator), Warawut Kittiwattanagul, Parnumas Piyavechvirat, Pojana Tapsai, Ajchara Namuang-jan (Khon Kaen Regional Hospital, 535); Upapat Chantapimpa (Chiangrai Prachanuko Hospital, 12); Chanothai Watanachai, Pusit Subsompon (Rayong Hospital, 11); Wipurat Pussanakawatin, Pensri Khunjan (Krabi Hospital, 10); Sakchai Tangchitvittaya, Somsak Nilapong (Suratthani Hospital, 8); Tanagorn Klangsang, Wibul Taechakosol (Roi Et Hospital, 2); Atirat Srinat (Lampang Hospital, 1).
Tunisia (63 patients): Zouheir Jerbi (national coordinator), Nebiha Borsali-Falfoul, Monia Rezgui (Hospital Habib Thameur, 63).
Turkey (2 patients): Nahit Cakar (Istanbul Medical Faculty, 2).
Uganda (43 patients): Hussein Ssenyonjo, Olive Kobusingye (Makerere Medical School, 43).
UK (1391 patients): Gabrielle Lomas, David Yates, Fiona Lecky (Hope Hospital, 209); Anthony Bleetman, Alan Baldwin, Emma Jenkinson, Shiela Pantrini (Birmingham Heartlands Hospital, 123); James Stewart, Nasreen Contractor, Trudy Roberts, Jim Butler (North Manchester General Hospital, 85); Alan Pinto, Diane Lee (Royal Albert Edward Infirmary, 83); Nigel Brayley, Karly Robbshaw, Clare Dix (Colchester General Hospital, 79); Sarah Graham, Sue Pye (Whiston Hospital, 69); Marcus Green, Annie Kellins (Selly Oak Hospital, 61); Chris Moulton, Barbara Fogg (Royal Bolton Hospital, 51); Rowland Cottingham, Sam Funnell, Utham Shanker (Eastbourne District General Hospital, 50); Claire Summers, Louise Malek (Trafford General Hospital, 41); Rowland Cottingham (national coordinator), Christopher Ashcroft, Jacky Powell (Royal Sussex County Hospital, 38); Steve Moore, Stephanie Buckley (Countess of Chester Hospital, 36); Mandy Grocutt, Steve Chambers (Worthing Hospital, 34); Amanda Morrice, Helen Marshall (Medway Maritime Hospital, 29); Julia Harris, Wendy Matthews, Jane Tippet (Chelsea and Westminster Hospital, 28); Simon Mardell, Fiona MacMillan, Anita Shaw (Furness General Hospital, 27); Pramod Luthra, Gill Dixon (Royal Oldham Hospital, 26); Mohammed Ahmed, John Butler, Mike Young (Stepping Hill Hospital, 26); Sue Mason, Ian Loveday (Northern General Hospital, 25); Christine Clark, Sam Taylor (Blackburn Royal Infirmary, 23); Paul Wilson (Cheltenham General Hospital, 23); Kassim Ali, Stuart Greenwood (Fairfield General Hospital, 23); Martin White, Rosa Perez (Queen Elizabeth the Queen Mother Hospital, 21); Sam Eljamel (Ninewells Hospital and Medical School, 19); Jonathan Wasserberg, Helen Shale (Queen Elizabeth Hospital Birmingham, 18); Colin Read, John McCarron (Russell’s Hall Hospital, 18); Aaron Pennell (Princess Alexandra Hospital, 16); Gautam Ray (Princess Royal Hospital, 14); John Thurston, Emma Brown (Darent Valley Hospital, 13); Lawrence Jaffey, Michael Graves (Royal Liverpool University Hospital, 12); Richard Bailey, Nancy Loveridge (Chesterfield and North Derbyshire Royal Hospital, 10); Geraint Evans, Shirleen Hughes, Major Kafeel Ahmed (Withybush General Hospital, 10); Jeremy Richardson, Claire Gallagher (Aberdeen Royal Infirmary, 8); Titus Odedun, Karen Lees (Ormskirk and District General Hospital, 8); David Foley, Nick Payne (Queen Mary’s Hospital, 8); Alan Pennycook, Carl Griffiths (Arrowe Park Hospital, 6); David Moore, Denise Byrne (City Hospital Birmingham, 5); Sunil Dasan (St Helier Hospital, 4); Ashis Banerjee, Steve McGuinness (Whittington Hospital, 4); Claude Chikhani (Doncaster Royal Infirmary, 2); Nigel Zoltie, Ian Barlow (Leeds General Infirmary, 2); Ian Stell (Bromley Hospital, 1); William Hulse, Jacqueline Crossley (Harrogate District Hospital, 1); Laurence Watkins (Institute of Neurology, 1); Balu Dorani (Queen Elizabeth Hospital Gateshead, 1).
Vietnam (2 patients)—Truong Van Viet (Cho Ray Hospital, 2).
Contributors: The writing committee comprised Pablo Perel (Chair), Miguel Arango, Tim Clayton, Phil Edwards, Edward Komolafe, Stuart Poccock, Ian Roberts, Haleema Shakur, Ewout Steyerberg, and Surakrant Yutthakasemsunt
Funding: The MRC CRASH trial was funded by the UK Medical Research Council .
Competing interests: None declared.
Ethical approval: All MRC CRASH collaborators obtained local ethics or research committee approval.
Provenance and peer review: Not commissioned; externally peer reviewed.
- ↵ Bruns J Jr, Hauser WA. The epidemiology of traumatic brain injury: a review. Epilepsia 2003 ; 44 (suppl 10): 2 -10. OpenUrl
- ↵ Fleminger S, Ponsford J. Long term outcome after traumatic brain injury. BMJ 2005 ; 331 : 1419 -20. OpenUrl FREE Full Text
- ↵ Hofman K, Primack A, Keusch G, Hrynkow S. Addressing the growing burden of trauma and injury in low- and middle-income countries. Am J Public Health 2005 ; 95 : 13 -7. OpenUrl CrossRef PubMed Web of Science
- ↵ Perel P, Wasserberg J, Ravi RR, Shakur H, Edwards P, Roberts I. Prognosis following head injury: a survey of doctors from developing and developed countries. J Eval Clin Pract 2007 ; 13 : 464 -5. OpenUrl CrossRef PubMed Web of Science
- ↵ Lee KL, Pryor DB, Harrell FE Jr, Califf RM, Behar VS, Floyd WL, et al. Predicting outcome in coronary disease. Statistical models versus expert clinicians. Am J Med 1986 ; 80 : 553 -60. OpenUrl CrossRef PubMed Web of Science
- ↵ Murray LS, Teasdale GM, Murray GD, Jennett B, Miller JD, Pickard JD, et al. Does prediction of outcome alter patient management? Lancet 1993 ; 341 : 1487 -91. OpenUrl CrossRef PubMed Web of Science
- ↵ Perel P, Edwards P, Wentz R, Roberts I. Systematic review of prognostic models in traumatic brain injury. BMC Med Inform Decis Mak 2006 ; 6 : 38 . OpenUrl CrossRef PubMed
- ↵ CRASH Trial Collaborators. Effect of intravenous corticosteroids on death within 14 days in 10 008 adults with clinically significant head injury (MRC CRASH trial): randomised placebo-controlled trial. Lancet 2004 ; 364 : 1321 -8. OpenUrl CrossRef PubMed Web of Science
- ↵ CRASH Trial Collaborators. Final results of MRC CRASH, a randomised placebo-controlled trial of intravenous corticosteroid in adults with head injury-outcomes at 6 months. Lancet 2005 ; 365 : 1957 -9. OpenUrl CrossRef PubMed Web of Science
- ↵ Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet 1975 ;i:480-4.
- ↵ World Bank. World development indicators . Washington, DC: World Bank, 2006 .
- ↵ Brain Trauma Foundation (US) AAoNS. Management and prognosis of severe traumatic brain injury . New York: 2000 . www.braintrauma.org/site/DocServer/Prognosis_Guidelines_for_web.pdf?docID=241 .
- ↵ Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996 ; 15 : 361 -87. OpenUrl CrossRef PubMed Web of Science
- ↵ Justice AC, Covinsky KE, Berlin JA. Assessing the generalizability of prognostic information. Ann Intern Med 1999 ; 130 : 515 -24. OpenUrl CrossRef PubMed Web of Science
- ↵ Maas AI, Marmarou A, Murray GD, Teasdale SG, Steyerberg EW. Prognosis and clinical trial design in traumatic brain injury: the IMPACT study. J Neurotrauma 2007 ; 24 : 232 -8. OpenUrl CrossRef PubMed Web of Science
- ↵ Hukkelhoven CW, Steyerberg EW, Rampen AJ, Farace E, Habbema JD, Marshall AF, et al. Patient age and outcome following severe traumatic brain injury: an analysis of 5600 patients. J Neurosurg 2003 ; 99 : 666 -73. OpenUrl CrossRef PubMed Web of Science
- ↵ Signorini DF, Andrews PJD, Jones PA, Wardlaw JM, Miller JD. Predicting survival using simple clinical variables: a case study in traumatic brain injury. J Neurol Neurosurg Psychiatry 1999 ; 66 : 20 -5. OpenUrl Abstract / FREE Full Text
- ↵ Maas AI, Hukkelhoven CW, Marshall LF, Steyerberg EW. Prediction of outcome in traumatic brain injury with computed tomographic characteristics: a comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery 2005 ; 57 : 1173 -82. OpenUrl CrossRef PubMed Web of Science
- ↵ Wardlaw JM, Easton VJ, Statham P. Which CT features help predict outcome after head injury? J Neurol Neurosurg Psychiatry 2002 ; 72 : 188 -92. OpenUrl Abstract / FREE Full Text
- ↵ Hukkelhoven CW, Steyerberg EW, Farace E, Habbema JD, Marshall LF, Maas AI. Regional differences in patient characteristics, case management, and outcomes in traumatic brain injury: experience from the tirilazad trials. J Neurosurg 2002 ; 97 : 549 -57. OpenUrl PubMed Web of Science
- ↵ Hukkelhoven CW, Steyerberg EW, Habbema JD, Farace E, Marmarou A, Murray GD, et al. Predicting outcome after traumatic brain injury: development and validation of a prognostic score based on admission characteristics. J Neurotrauma 2005 ; 22 : 1025 -39. OpenUrl CrossRef PubMed Web of Science
- ↵ Altman DG. Systematic reviews in health care: Systematic reviews of evaluations of prognostic variables. BMJ 2001 ; 323 : 224 -8. OpenUrl FREE Full Text
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Current issue
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 19, Issue 6
- The MRC CRASH Trial: study design, baseline data, and outcome in 1000 randomised patients in the pilot phase
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
The CRASH Trial Pilot Study Collaborative Group
- Correspondence to: Mr P Edwards, CRASH Trial Co-ordinating Centre, London School of Hygiene and Tropical Medicine, 49–51 Bedford Square, London WC1B 3DP, UK; Phil.Edwards{at}LSHTM.ac.uk
Objectives: To test the design and feasibility of a large scale multicentre randomised controlled trial evaluating the efficacy and safety of a high dose corticosteroid infusion after head injury. To assess whether large numbers of patients could be enrolled and treated within eight hours from injury and then followed up at six months.
Methods: Randomised placebo controlled multicentre trial of a 48 hour corticosteroid infusion after significant head injury. All head injured adults who were observed while in hospital to have GCS of 14 or less (out of a maximum score of 15), and who were within eight hours of the injury, were eligible for trial entry. Analysis of baseline and outcome data (for both treatment groups combined) for 1000 patients enrolled in the pilot phase of the MRC CRASH Trial.
Results: Fifty two hospitals in 14 countries participated in the pilot phase, recruiting an average of one patient per hospital per month. Of the 1000 randomised patients, 330 (33%) had mild head injury, 289 (29%) had moderate head injury, and 381 (38%) had severe head injury. Seven hundred and nine (71%) patients were randomised within three hours of injury. Outcome at two weeks from injury was known for 991 (99%) patients, of whom 170 (17%) patients died. At the time of writing, six month follow up for the first 500 patients was nearly complete. Vital status was known for 465 (93%) of the 500 patients, of whom 97 (21%) had died. Functional status based on the Glasgow Outcome Scale was known for 438 (88%) of the 500 patients: 21% were dead, 17% were severely disabled, 22% were moderately disabled, and 34% had made a good recovery.
Conclusions: The trial procedures proved practicable and a wide variety of patients were recruited in the emergency department within eight hours of injury. Using simple outcome measures, large numbers of patients can be successfully followed up.
- head injury
- corticosteroid infusion
- randomised controlled trial
- pilot phase
https://doi.org/10.1136/emj.19.6.510
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Worldwide more than a million people die from head injuries each year and a similar number are disabled, often with profound effects on the quality of life of the affected individuals and their carers. 1 In 1990 over 5 500 000 intracranial injuries were caused by road traffic crashes alone. Road traffic crashes account for most of the deaths and serious head injuries and car use is rapidly increasing in many countries. The identification of effective treatments for head injury is of global health importance.
Corticosteroids have been used in the treatment of head injury for over 30 years, although recently their value has been questioned because of the failure to reliably demonstrate effectiveness in randomised trials. 2 For such a common problem, if a treatment as simple as short-term corticosteroids produced just a moderate benefit, this could be worthwhile. However, existing randomised trials of corticosteroids in head injury have been too small to demonstrate or refute the possibility of moderate but clinically important benefits or harm from corticosteroids. 3 To determine reliably the effects of a high dose corticosteroid infusion on death and on disability after significant head injury, many thousands of acute head injury patients must be randomised between corticosteroid and placebo infusions. 4, 5
Axonal disruption can continue for several hours after acute central nervous system trauma. In animal studies, it has been shown that early administration of corticosteroids is important for maximal effect. 6 Hence there may be an early phase when further neurological deterioration can be prevented. Timing of corticosteroid administration is also considered to be important in acute spinal cord injury. 7 The aim of the CRASH Trial pilot phase was to test the design and feasibility of a large scale multicentre trial to evaluate the effectiveness and safety of a short-term high dose corticosteroid infusion after significant head injury. 5 In particular, the pilot phase sought to assess whether the enrolment and follow up of large numbers of head injured patients would be possible, and whether treatment could be started within eight hours from the time of injury.
Eligibility
All head injured patients (judged to be 16 years or older) who were observed while in hospital to have a score on the Glasgow Coma Scale of 14 or less (out of a maximum score of 15), and who were within eight hours of injury, were eligible for trial entry. There was no other pre-specified exclusion criterion, as the fundamental eligibility criterion was the responsible doctor's “uncertainty” whether or not to use corticosteroids in a particular adult with head injury.
Patients with head injury and impaired consciousness may be unable to give properly informed consent. In emergency situations it may not be medically appropriate to delay the start of treatment until proxy consent can be obtained, doctors therefore took responsibility for entering their patients, just as they would take responsibility for choosing other treatments. However, the specific requirements of local research ethics committees were strictly adhered to at all times. Information leaflets about the study were provided for patients and their friends and relatives.
Treatment regimen
The study treatment was a 48 hour infusion of methylprednisolone (MP) or placebo. The loading dose was 2 g MP (or matching placebo) over one hour in a 100 ml infusion. The maintenance dose was 0.4g/hour for 48 hours in a 20 ml per hour infusion (or matching placebo). If clinically appropriate, patients could be discharged from hospital before completing the trial treatment.
Randomisation, baseline data collection, and emergency unblinding
Patients were randomised in one of two ways. Central randomisation was by telephoning the 24 hour randomisation service at the Clinical Trial Service Unit (CTSU), Oxford, UK. During the telephone call, baseline data were requested and then entered into the CTSU database. The baseline data collected were—sex, age, hours since injury, Glasgow Coma Scale (GCS) including eye opening (scale 1–4), motor response (scale 1–6), and verbal response (scale 1–5), and localising signs as judged by the presence of one or more non-reactive pupils. A reasonable balance with respect to these, and with respect to hospital, was achieved using the method of treatment allocation known as “minimisation”. 8 The minimisation factors were: sex, age (16–24 years, 25–34 years, 35 years and older), hours since injury (one hour or less, one to three hours, over three hours), GCS (3–8, 9–12, 13–14), localising signs, hospital and country. After recording the baseline data, the CTSU computer allocated a number identifying one of the treatment packs stored in the emergency department. Non-central randomisation was used in countries where telephone access is not reliable, or where hospital staff could not easily speak English. For these patients, blinded treatment packs were used sequentially and the baseline data were immediately sent by fax, or as encrypted email attachments to the Co-ordinating Centre. These data were entered into the CTSU database on the day of receipt. Only in special circumstances could treatment allocation be unblinded. Where emergency unblinding was necessary, for example when doctors believed that clinical management depended importantly on knowing whether corticosteroid or placebo had been given, unblinding was possible by telephoning the randomisation service, giving the patient details.
Outcome data collection
Inhospital deaths, complications, and short-term recovery were recorded on a single sided form completed by the responsible doctor at death, discharge, or two weeks after injury, whichever occurred first. Long term recovery was assessed at six months by a simple postal questionnaire, sent to patients or their carers, or by telephone interviews or by home visits (Sri Lanka and South Africa only). Before the start of the pilot, a simple postal questionnaire version of the Glasgow Outcome Scale (GOS) was developed and found to be both reliable and valid. 9 We also conducted a systematic review of randomised controlled trials of strategies to influence response to postal questionnaires in order to identify ways to maximise response to the follow up questionnaire. 10, 11 The postal version of the GOS was translated into the relevant languages with back translation to ensure accuracy. Completed questionnaires were returned to the Co-ordinating Centre.
Data processing, analysis, and the data monitoring and ethics committee
Baseline data were transferred electronically each day from CTSU to the Co-ordinating Centre. Outcome data were entered twice and any differences resolved to eliminate data entry errors. Further extensive checks for data consistency were also undertaken. The pre-specified principal analyses were the effects of treatment on death from any cause within two weeks of injury, and on death or dependence at six months. The independent Data Monitoring and Ethics Committee periodically reviewed the accumulating trial data in strict confidence. In May 2000 the Committee reviewed the data and reported that the available data provided no cause to recommend any change to the study procedures or the plans for the expansion of patient recruitment in the main phase of the trial. As the treatment regimen and allocation remained unchanged in the main phase of the trial, the pilot phase results by treatment allocation will be combined with those of the main phase of the study, and will therefore remain confidential until the completion of the trial.
The first patient was enrolled on 13 April 1999, and by 21 December 2000 a total of 1000 patients had been enrolled, an average recruitment of 50 patients per month.
Fifty two hospitals in 14 countries participated in the pilot phase, recruiting an average of 1.0 (range 0.1–6.3) patient per hospital per month.
Baseline characteristics
Table 1 shows the baseline characteristics for the 1000 patients by treatment status. There were 781 (78%) men and 219 (22%) women. The average age was 39 years (SD=19, median = 35), with a range from 14 to 99 years. Four patients were aged under 16 years but were judged to have been aged 16 at the time of randomisation. Figure 1 shows the distribution of patient ages at randomisation. The median time from injury to randomisation was two hours. Figure 2 shows the distribution of time from injury. Four hundred and four (40%) patients were randomised within one hour, 305 (30%) patients were randomised between one and three hours, and 291 (29%) patients were randomised between three and eight hours of injury. Severity of head injury was classified as mild (GCS 13 and 14) in 330 (33%) patients, moderate (GCS 9 to 12) in 289 (29%) patients, and severe (GCS 3 to 8) in 381 (38%) patients. Patients were classified as having localising signs only if one or both of their pupils were not reactive to light. One hundred and fifty six (16%) patients had localising signs before randomisation.
- View inline
Baseline characteristics for 1000 patients by treatment status
- Download figure
- Open in new tab
- Download powerpoint
Age of patients at randomisation.
Time between injury and randomisation.
Principal measures of efficacy: deaths within 14 days, death or dependence at six months and proportion of patients with an unfavourable outcome
Table 2 shows the characteristics of the patients at the time of randomisation with their outcome at two weeks. Early outcome forms were received for 991 (99%) of the 1000 randomised patients. One hundred and seventy (17%) patients died within two weeks of randomisation. In assessing long term recovery, the follow up of some patients took over nine months to achieve. For this reason, we have presented the results for outcome at six months for the first 500 patients only. Table 3 shows the characteristics of these patients with their outcome at six months (we continue to seek the information for patients for whom long term recovery is still not known). Among the first 500 patients, vital status at six months was known for 465 (93%), of whom 97 (21%) had died. Functional status at six months based on the GOS was known for 438 (88%) of the 500 patients: 21% were dead, 17% were severely disabled, 22% were moderately disabled, and 34% had made a good recovery. Thus at six months 38% of patients had an unfavourable outcome (severely disabled, persistent vegetative state, or dead).
Outcome at two weeks by baseline characteristics
Outcome at six months by baseline characteristics
Other measures of efficacy and safety (fatal and non-fatal events within 14 days)
Table 4 shows the aspects of patient management and health events that were explicitly sought on the early outcome form (figures are for both treatment groups combined). Four hundred and twenty four patients were admitted to ICU (43%), 241 (57%) of whom were admitted for four days or more. Seizure was recorded for 176 (18%) patients, haematemesis or melaena for four (0.4%) patients, wound infections for 31 (3%) patients, pneumonia treated with antibiotics for 90 (9%) patients, other treatment with antibiotics for 286 (29%) patients, neurosurgical operation for 195 (20%) patients, and major extracranial injury for 179 (18%) patients. Head CT scans were performed on 754 (76%) patients. The first head CT scan result was normal in 204 (27%) patients. Among the scans showing some abnormality, there were 76 (10%) with obliteration of the third ventricle or basal cisterns, 96 (13%) with midline shift over 5 mm, 163 (22%) with a subarachnoid bleed and 280 (37%) with a haematoma.
Patient management and health events within14 days of randomisation
Compliance with trial treatment
Compliance with the trial treatment was known for 941 (94%) patients, of whom 918 (98%) received the full loading dose. When clinically appropriate, patients were discharged from hospital before completing the full maintenance dose. Two hundred and six patients (22%) were discharged before completing the maintenance dose and received less than 24 hours of treatment.
Emergency unblinding
Of the 1000 patients randomised, the treatment allocation was unblinded for seven (0.7%) patients. The most common reason for emergency unblinding, accounting for four instances, was that after randomisation the patient was found to have a spinal cord injury for which the responsible doctor considered that corticosteroids were indicated.
The CRASH Trial is the first very large scale randomised controlled trial in head injury, aiming to recruit and follow up 20 000 patients by 2005. The pilot phase confirmed that the study design was practicable in both university and general hospitals, and in high and middle income countries. Sample size calculations for the CRASH Trial were based on an estimated risk of death among controls of 15%. These data were obtained from a recent randomised controlled trial in head injury using similar but not identical inclusion criteria. 12 The pilot phase provides a more precise estimate of this parameter, using the uncertainty principle as the fundamental eligibility criterion, and confirms that the estimated event rate was appropriate (17% dead within two weeks of injury). The pilot study results at two weeks and at six months confirm that the limited data collected in the CRASH Trial can separate groups at high risk and low risk of a poor outcome. The main predictors of poor outcome were age and score on the GCS.
Among the first 1000 patients enrolled in the CRASH Trial, the median time from injury to randomisation was two hours. Over 70% of patients were randomised within three hours of injury, confirming that the treatment of head injured patients within an appropriate therapeutic period is feasible.
In a recent survey of previous randomised controlled trials in head injury the average number of participants per trial was 82 and the average loss to follow up was 19%. 13 In the pilot phase of the CRASH Trial, short-term outcome was known for 99% of the 1000 randomised participants, and long term outcome was known for 93% of the first 500. This confirms that with simple outcome measures using postal strategies that have been shown to increase response, large numbers of patients can be successfully followed up. Tracing and contacting patients or their carers to assess long term recovery can take many months to achieve. However, we continue to be successful in tracing patients for whom long term recovery is not known, and continue to make efforts to collect this information.
The CRASH Trial is now the largest head injury trial ever conducted. Participation by more accident and emergency, intensive care, neurosurgical, and trauma departments is still needed worldwide. The Trial Co-ordinating Centre will supply the documentation needed to apply for ethical approval and all materials needed to take part, once approval is obtained. The full trial protocol is available from CRASH Co-ordinating Centre, London School of Hygiene and Tropical Medicine, 49–51 Bedford Square, London WC1B 3DP (email CRASH{at}LSHTM.ac.uk ), or alternatively visit the CRASH web site ( www.CRASH.LSHTM.ac.uk ).
Contributors
Belgium (116 patients): A Z Kliena Hospital, Dr V Braet; Centre Hospitalier Regional de Namur, Dr G Mazairac; Hospital of Jolimont, Dr J Jacques. Colombia (8): Clinica Las Americas, Dr MF Arango. Czech Republic (181): Research Institute for Traumatology and Surgery of Brno, Professor P Svoboda, Dr J Ochmann, Dr I Kantorova, Dr P Scheer; Hospital Pribram, Dr J Bürger; Masaryk Hospital Usti n/L, Dr K Edelmann; Neurosurgical Clinic Charles University Hospital Prague, MUDr F Kramar. Eire (24): St James's University Hospital, Mr P Plunkett, Dr G MacMahon. Germany (1): Ernst Moritz Arndt University, Professor M Gaab, Dr W Kleist. Greece (3): KAT Hospital of Athens, Dr P Myrianthefs. Malaysia (4): Hospital University Science Malaysia, Dr J Abdullah, Dr G Ghazaime, Dr A Ariff, Dr M Abdullah, Dr I Zamzuri, Dr A Saufi, Dr A Ghani. Singapore (1): National Neuroscience Institute, Mr J Tan. Slovakia (20): Reimann Hospital, MD S Trenkler, Dr R Nachaj. South Africa (65): Tygerberg Academic Hospital, Professor B Hartzenberg; Wentworth Hospital, Dr N Nathoo, Mr S Khumalo. Spain (24): Trauma Centre - Virgen Del Rocio, Dr MA Muñoz-Sánchez, Dr JM Flores-Cordero, Dr F Murillo. Sri Lanka (62): Batticaloa General Hospital, Ms V Laloë (Médecins Sans Frontiéres); National Hospital of Sri Lanka, Dr S Perera. Switzerland (30): University Hospital of Zurich, Professor R Stocker, Miss S Ludwig, Miss A Sauerland. United Kingdom (461): Aberdeen Royal Infirmary, Mr J Richardson, Sr C Gallagher; Arrowe Park Hospital, Mr A Pennycook; Birmingham Heartlands Hospital, Mr A Bleetman, Sr S Pantrini; Blackburn Royal Infirmary, Dr C Clark, SN S Riding; Bromley Hospital, Dr I Stell; Bury General Hospital, Dr K Ali, CN S Greenwood; Cheltenham General Hospital, Mr P Wilson, Sr L Iddles; City Hospital, Dr D Moore, Dr K Murali; Colchester General Hospital, Mr N Brayley, Mr O Taha El-Amin, SN J Lynch; Countess of Chester Hospital, Mrs M Waters, Sr J Sampson; Doncaster Royal Infirmary, Mr C Chikhani; Eastbourne District General Hospital, Mr R Cottingham, Mr S Funnell; Furness General Hospital, Dr S Mardel, SN A Shaw, SN W Waddington; Harrogate District Hospital, Dr W Hulse, Sr J Crossley; Hope Hospital, Sister G Lomas, Professor D Yates; Leeds General Infirmary, Mr N Zoltie, Mr R Lewis; Medway Maritime Hospital, Dr A Morrice, Miss C Allonby-Neve; Ninewells Hospital and Medical School, Mr S Eljamel; North Manchester General Hospital, Mr J Stuart, SN T Roberts; Northern General Hospital, Dr S Mason, Mr I Loveday; Princess Alexandra Hospital, Mr A Saha, Mr A Pennell, CN D McPhee; Queen Elizabeth Hospital, Birmingham, Mr J Wasserberg, Sr H Shale; Queen Elizabeth Hospital, Gateshead, Mr B Dorani; Queen Elizabeth The Queen Mother Hospital, Mr A Jones, Sr R Perez; Royal Albert Edward Infirmary, Mr A Pinto, Sr D Lee; Royal Bolton Hospital, Dr B Flavin, Mrs B Fogg; Royal Liverpool University Hospital, Dr L Jaffey, Mr M Graves; Royal Oldham Hospital, Mr P Luthra, Sr G Dixon; Selly Oak Hospital, Mr M Green, Sr J Smith; Stepping Hill Hospital, Dr S Southworth, Dr J Butler, Mr M Young; Trafford General Hospital, Miss C Summers, Sr L Katiff; Whiston Hospital, Dr S Graham, Dr T Brown, Sr S Bathgate.
The CRASH Co-ordinating Centre (London)
Professor I Roberts (Clinical Co-ordinator); Miss N Ritchie (Trial Manager); Mr P Edwards (Senior Research Fellow); Ms R Mashru (Data Manager); Ms J Fernandes, Ms C Godward (Follow-up Co-ordinators); Mrs M Ramos (Trial Administrator); Mr A Ritchie (Trial Programmer).
The CRASH Trial Management Group
Professor D Yates; Professor G Teasdale, Ms B Farrell; Mr J Wasserberg; Professor I Roberts; Professor P Sandercock; Ms G Lomas; Miss N Ritchie; Mr P Edwards.
Clinical Trial Service Unit, Oxford University
Professor R Peto, Dr A Young, Mr P Dove.
Data Monitoring Committee
Professor R Collins, Professor S MacMahon (Chair), Professor S Haines, Professor C Warlow.
Writing Committee
Mr P Edwards, Ms B Farrell, Ms G Lomas, Ms R Mashru, Miss N Ritchie, Professor I Roberts, Professor P Sandercock, Mr J Wasserberg, Professor D Yates.
Mr P Edwards and Professor I Roberts.
- ↵ Murray CJ , Lopez AD. Global health statistics: a compendium of incidence, prevalence and mortality estimates for over 200 conditions. Harvard School of Public Health, Boston: Harvard University Press, 1996.
- ↵ Bullock R , Chesnut RM, Clifton G, et al . Part 1: Guidelines for the management of severe traumatic brain injury. J Neurotrauma 2000 ; 17 : 499 –553. OpenUrl
- ↵ Alderson P , Roberts I. Corticosteroids in acute traumatic brain injury: a systematic review of randomised trials. BMJ 1997 ; 314 : 1855 –9. OpenUrl Abstract / FREE Full Text
- ↵ Teasdale G . The treatment of head trauma: implications for the future. J Neurotrauma 1991 ; 8 (suppl 1): 53 –60. OpenUrl
- ↵ Yates D , Farrell B, Teasdale G, et al . Corticosteroids in head injury—the CRASH trial. J Accid Emerg Med 1999 ; 16 : 83 –4. OpenUrl FREE Full Text
- ↵ Braughler JM , Hall ED. Current application of “high-dose” steroid therapy for CNS injury—a pharmacological perspective. J Neurosurg 1985 ; 62 : 806 –10. OpenUrl PubMed Web of Science
- ↵ Bracken MB . Pharmacological interventions for acute spinal cord injury (Cochrane Review). In: The Cochrane Library, Issue 4, 1999 . Oxford: Update Software.
- ↵ White S , Freedman L. Allocation of patients to treatment groups in a controlled clinical study. Br J Cancer 1978 ; 37 : 849 –57. OpenUrl PubMed Web of Science
- ↵ Wilson L , Edwards P, Fiddes H, et al . Reliability of postal questionnaires for the Glasgow Outcome Scale. J Neurotrauma (In press).
- ↵ Edwards P , Roberts I, Clarke M, et al . Methods to influence response to postal questionnaires (Cochrane Methodology Review). In: The Cochrane Library, Issue 2, 2002. Oxford: Update Software.
- ↵ Edwards P , Roberts I, Clarke M, et al . Increasing response rates to postal questionnaires: systematic review. BMJ 2002 ; 324 : 1183 –5. OpenUrl Abstract / FREE Full Text
- ↵ Gaab MR , Trost HA, Alcantara A, et al . Ultrahigh dexamethasone in acute brain injury. Results from a prospective randomised double-blind multi-centre trial. Zentralbl Neurochir 1994 ; 55 : 135 –43. OpenUrl PubMed
- ↵ Dickinson K , Bunn F, Wentz R, et al . Size and quality of randomised controlled trials in head injury: review of published studies. BMJ 2000 ; 320 : 1308 –11. OpenUrl Abstract / FREE Full Text
Conflicts of interest: The CRASH Trial is sponsored by the Medical research Council (MRC) and not the manufactures of methyprednisolone. MRC funding covers meetings and central organisational costs only. Pharmacia Corporation are donoting drug and placebo, but the design, management, and finance of the study are entirely independent of them.
Linked Articles
- Primary Survey Primary Survey Pete Driscoll Jim Wardrope Emergency Medicine Journal 2002; 19 488-488 Published Online First: 01 Nov 2002. doi: 10.1136/emj.19.6.488-a
Read the full text or download the PDF:
The MRC CRASH trial--a large, simple randomised trial of steroids in head injury
Affiliation.
- 1 Department of Neurosurgery, Queen Elizabeth Hospital, Birmingham, United Kingdom. [email protected]
- PMID: 15335109
- DOI: 10.1007/978-3-7091-0603-7_15
CRASH (Corticosteroid randomisation after significant head injury) is a prospective multi-centre randomised double blind study of methylprednisolone versus placebo in mild, moderate and severe head injury. Patients are eligible up to 8 hours from injury. To date the CRASH trial has recruited 9000 patients. The trial is recruiting from 200 hospitals in 50 countries with another 100 centres planning to join the trial. The target for recruitment is 20,000 patients by 2006. The trial is wholly funded by the Medical Research Council of Great Britain and is multidisciplinary, involving doctors and nurses from a range of specialities. A recent systematic review of corticosteroids in head injury demonstrated a risk of death in the corticosteroid treated group 2% lower than in the control group. The 95% confidence interval ranges from a 60%, lower mortality to a 2% higher mortality. This result is compatible with there being no real benefit, but it is also compatible with there being a small benefit of a few percent. An improvement in mortality of 2% would theoretically save 10,000 lives per 500,000 patients treated. The global impact of such a treatment effect would be significant as the number of head injuries world-wide continues to rise.
Publication types
- Clinical Trial
- Multicenter Study
- Randomized Controlled Trial
- Craniocerebral Trauma / drug therapy*
- Craniocerebral Trauma / mortality*
- Double-Blind Method
- Glucocorticoids / therapeutic use*
- International Cooperation
- Methylprednisolone / therapeutic use*
- Neuroprotective Agents / therapeutic use*
- Randomized Controlled Trials as Topic / methods*
- Recovery of Function
- Treatment Outcome
- Glucocorticoids
- Neuroprotective Agents
- Methylprednisolone
- Original research
- Open access
- Published: 17 July 2021

Selection of CT variables and prognostic models for outcome prediction in patients with traumatic brain injury
- Djino Khaki 1 , 2 ,
- Virpi Hietanen 3 ,
- Alba Corell ORCID: orcid.org/0000-0002-6329-2392 1 , 2 ,
- Helena Odenstedt Hergès 3 , 4 &
- Johan Ljungqvist 1 , 2
Scandinavian Journal of Trauma, Resuscitation and Emergency Medicine volume 29 , Article number: 94 ( 2021 ) Cite this article
3675 Accesses
12 Citations
4 Altmetric
Metrics details
Traumatic brain injuries (TBI) are associated with high risk of morbidity and mortality. Early outcome prediction in patients with TBI require reliable data input and stable prognostic models. The aim of this investigation was to analyze different CT classification systems and prognostic calculators in a representative population of TBI-patients, with known outcomes, in a neurointensive care unit (NICU), to identify the most suitable CT scoring system for continued research.
Materials and methods
We retrospectively included 158 consecutive patients with TBI admitted to the NICU at a level 1 trauma center in Sweden from 2012 to 2016. Baseline data on admission was recorded, CT scans were reviewed, and patient outcome one year after trauma was assessed according to Glasgow Outcome Scale (GOS). The Marshall classification, Rotterdam scoring system, Helsinki CT score and Stockholm CT score were tested, in addition to the IMPACT and CRASH prognostic calculators. The results were then compared with the actual outcomes.
Glasgow Coma Scale score on admission was 3–8 in 38%, 9–13 in 27.2%, and 14–15 in 34.8% of the patients. GOS after one year showed good recovery in 15.8%, moderate disability in 27.2%, severe disability in 24.7%, vegetative state in 1.3% and death in 29.7%. When adding the variables from the IMPACT base model to the CT scoring systems, the Stockholm CT score yielded the strongest relationship to actual outcome. The results from the prognostic calculators IMPACT and CRASH were divided into two subgroups of mortality (percentages); ≤50% (favorable outcome) and > 50% (unfavorable outcome). This yielded favorable IMPACT and CRASH scores in 54.4 and 38.0% respectively.
The Stockholm CT score and the Helsinki score yielded the closest relationship between the models and the actual outcomes in this consecutive patient series, representative of a NICU TBI-population. Furthermore, the Stockholm CT score yielded the strongest overall relationship when adding variables from the IMPACT base model and would be our method of choice for continued research when using any of the current available CT score models.
Traumatic brain injury (TBI) is a major cause of death and disability worldwide [ 1 , 2 , 3 ]. Risk stratification and early outcome prediction is important for triage and clinical decision making as well as for communication with the patients’ relatives and for clinical audit purposes. The need for accurate outcome prediction has opened the field of prognostic modeling in TBI. Historically, prognostication relied merely on clinical information, and although this is still important, more complex models also include information about radiological features, laboratory results and biomarkers [ 4 , 5 ]. The most commonly cited models, CRASH score [ 6 ] and IMPACT score [ 7 ], combine clinical and radiological information to make individual prognoses.
Studies of these models have identified four important prognostic variables following TBI: pupillary response, Glasgow Coma Scale (GCS) score, patient age, and radiological findings on computed tomography (CT) [ 7 ]. These prognostic features have been displayed graphically, enabling risk stratification in relation to outcome, and ultimately, provide a tool to aid in clinical decision making [ 8 , 9 ]. The CRASH and IMPACT models are continuously validated on new patient series [ 10 , 11 , 12 , 13 , 14 ].
The CRASH trial was an international multicenter study which aimed to determine the effects on death and disability of a short-term corticosteroid infusion following significant head injury, and the first results were published in 2004. However, this trial eventually led to the presentation of a prognostic calculator in TBI [ 6 ]. The International Mission on Prognosis and Analysis of randomized Controlled Trials in TBI (IMPACT) studies were initiated in 2003 and continued over a 10-year period. A collaboration that commenced with the Medical Research Council of Great Britain (MRC), CRASH, led to the presentation of an enhanced prognostic calculator that included predictors of outcome in three prognostic models of increasing complexity; a Core model based on injury severity and demographics (including age, motor score and pupillary reactivity), an Extended model additionally including CT information (based on Marshall CT classification) and secondary insults (hypoxia and hypotension); and a Lab model additionally including glucose and hemoglobin values [ 15 ]. The results of CRASH and IMPACT prognostic calculators are presented as the predicted probability in percent of 6 month mortality and unfavourable outcome, where 0% responds to no risk of mortality or unfavourable outcome and 100% an extremely high risk of mortality and unfavourable outcome.
However, several other investigations have aimed to improve the classification of the CT variables, and for this study, four other CT classification scores were included in addition to the CRASH score and the IMPACT score. These scores were the Marshall CT classification [ 16 ], the Rotterdam scoring system [ 17 ], the Helsinki CT score [ 18 ] and the Stockholm CT score [ 19 ] (see Supplementary Table 1 for an overview). While the Marshall CT classification has been considered “gold standard”, albeit limitations, the Rotterdam scoring system was based on Marshall CT classification with the addition of other CT variables for a better estimated prognosis [ 17 ]. The Helsinki CT score and Stockholm CT score are similar to the previous mentioned scores, with their own individual variables [ 18 , 19 ].
The aim of this investigation was to test and analyze different CT classification systems and prognostic calculators in a representative population of TBI-patients, with known outcomes, in a neurointensive care unit (NICU), to identify the most suitable CT scoring system for clinical use and continued research.
Patient cohort
All consecutive adult patients with TBI, admitted to the neuro-intensive care unit (NICU) at the level 1 trauma center at Sahlgrenska University Hospital, in Gothenburg, Sweden from 2012 to 2016 were identified ( n = 188), and 158 patients were included. Exclusion criteria were subacute injury, missing person data, patient transferred from another neurotrauma center and penetrating injury, see Fig. 1 . Baseline data and clinical variables were retrieved from the medical records. These variables included: date of trauma, gender, age, trauma mechanism, GCS score, pupillary response, best motor response, signs of hypoxia or hypotension, blood glucose levels, hemoglobin levels (Hb), any signs of extracranial injuries, and whether or not the patient had any anticoagulatory medications.
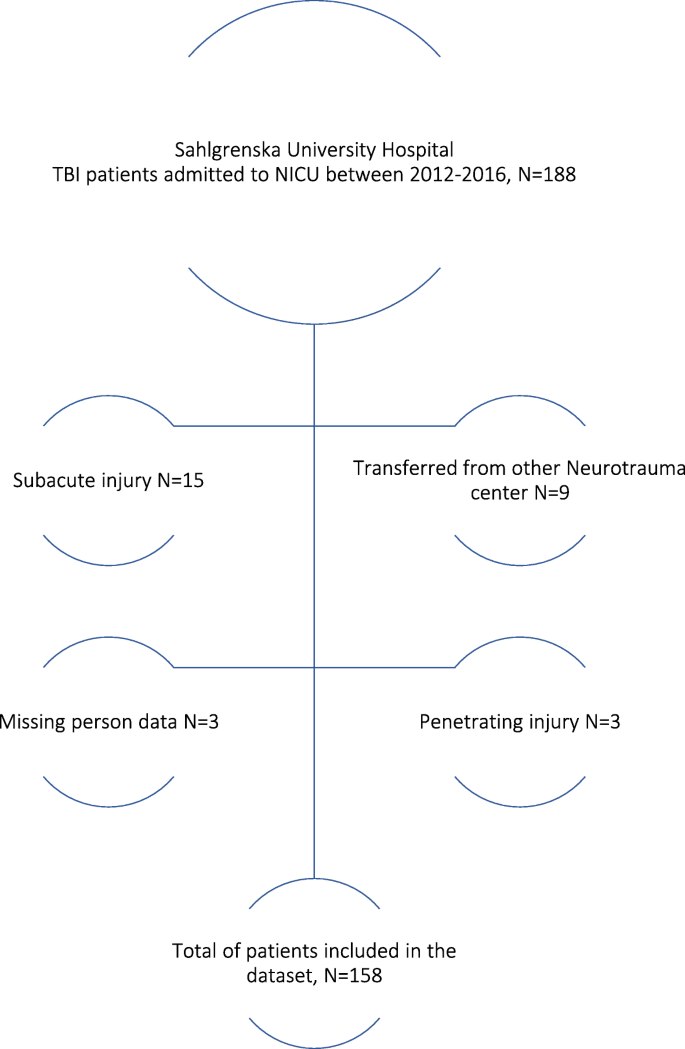
Flow-chart of inclusion and exclusion of patients
The trauma mechanisms were classified according to the categories fall from height, assault, unclear reason of trauma (where the patient was found unconscious), and traffic accidents. Any extracranial injuries which by themselves required hospital care (in addition to the intracranial injuries) were also registered in the data material. These could include injuries to the upper and lower limbs, vertebral column, thoracic or abdominal injuries as well as facial injuries.
The traffic accidents included any accidents where a vehicle (car, bus, tram, motorcycle, bicycle, or trailer) either collided with another vehicle, with a person, or as a single vehicle accident.
Patients initially admitted to local hospitals in the area who were in need of neurointensive care were transferred to the level 1 trauma center at Sahlgrenska University Hospital and the NICU. After treatment, the patients were again transferred back to their local hospital for continued care and rehabilitation.
Radiological CT scores and prognostic models
CT examinations were reviewed, and data for the variables of the different CT scores was registered. The first CT scans upon arrival were retrieved rather than the “worst” CT scans. The Marshall CT classification, Rotterdam scoring system, Helsinki and Stockholm CT scores were tested, and the IMPACT and CRASH prognostic calculators were applied [ 7 ].
The results of the CRASH and IMPACT calculators are presented as continuous variables from 0 to 100%, where 0% represents no risk for mortality or unfavorable outcome at 6 months and 100% a guaranteed risk for mortality or unfavorable outcome at 6 months. For this study, we dichotomized the predicted outcomes, i.e. ≤50% was assessed as favorable outcome (GOS 4–5), and > 50% was assessed as unfavorable outcome GOS [ 1 , 2 , 3 ].
Glasgow Outcome Scale (GOS) was used for global evaluation of outcome. GOS is a grading scale ranging from 1 (dead) to 5 (full recovery). An unfavorable outcome was defined as GOS 1–3 and favorable outcome as GOS 4–5. The assessment of GOS scores was based on interviews with the patients or their next of kin by a specially trained intensive care nurse (VH) one year post injury [ 20 ].
The statistical analyses were made using statistical software SPSS, version 25. Statistical significance was set at p < 0.05. The categorical variables were analyzed by frequencies and cross tables which are presented in numbers and percentages. The continuous variables are presented as medians and interquartile ranges due to non-normal distributed data. To show the correlation between the CT scoring systems and GOS, mean plots were used. The plots were made by variance analyses (one-way ANOVA). For validation and comparison of the different CT scoring systems, we used two statistical methods; logistical regression analyses and area under the receiver operating characteristic curve (AUC).
Logistical regression analyses were used to measure the association and accuracy between the different CT scoring systems and GOS. This yielded Nagelkerke’s pseudo-R2 which in turn gave a value between 0 and 1, where 0 equals no explanatory variation between the outcome and the model, and 1 equals fully explanatory variation.
Furthermore, AUC was used to compare with the measures of Nagelkerke’s pseudo-R2 which also gives a value of the variance between the outcome and the model. AUC gave a value between 0.5–1, where 0.5 indicates nothing more than random chance and 1 indicates a perfect association. To be able to perform the aforementioned analyses, GOS score was dichotomized in two ways. First GOS 1–3 versus 4–5 (unfavorable versus favorable outcome) and GOS 1 versus GOS 2–5 (dead versus alive).
Finally, a multivariable analysis by logistic regression was performed to further indicate the individual CT scoring systems’ value when added to the IMPACT base model. These variables included pupillary response, glucose, Hb level, age, and GCS on admission.
This study has been approved by the Swedish Ethical Review Authority (2019–04330). Formal consent from the patients was waived by the Review Authority.
158 patients with TBI were included during the study period, see Fig. 1 . The mean age was 59 years and 70% were male. Fall from heights and traffic accidents were the most common trauma mechanisms (55 and 22%, respectively). The demographics, CT variables and outcomes are presented in Table 1 .
GCS scores were assessed upon admission, and the patients were divided into three groups depending on total score; severe TBI (GCS 3–8), moderate TBI (GCS 9–13) and mild TBI (GCS 14–15). In total, 60 patients presented with a severe TBI (38%), 43 patients with moderate TBI (27%) and 55 patients (35%) with mild TBI. Acute subdural hematomas were the most predominant finding present in 85% of the patients. The results from the prognostic calculators IMPACT and CRASH yielded a favorable IMPACT score in 54.4% of the cohort, and a favorable CRASH score in 38.0%. GOS at one year after discharge showed good recovery in 15.8%, moderate disability in 27.2%, severe disability in 24.7%, and death in 29.7%. See Table 1 .
All CT scores, as well as the IMPACT and CRASH prognostic models, were tested in relation to the actual outcomes of the patients. The relationships between CT scores, prognostic models and outcome, according to GOS, are given in Fig. 2 A-D. To illustrate the relationship between the different CT scoring systems, i.e., Stockholm (A), Helsinki (B), Rotterdam (C), Marshall (D), with outcome (GOS), means plots were used where the y-axis shows the CT scoring system, and the x-axis shows GOS. This illustrates that the higher the CT scoring value, the worse the outcome and vice versa. The relationships between GOS and IMPACT and CRASH, respectively, are illustrated in Fig. 3 A-B.
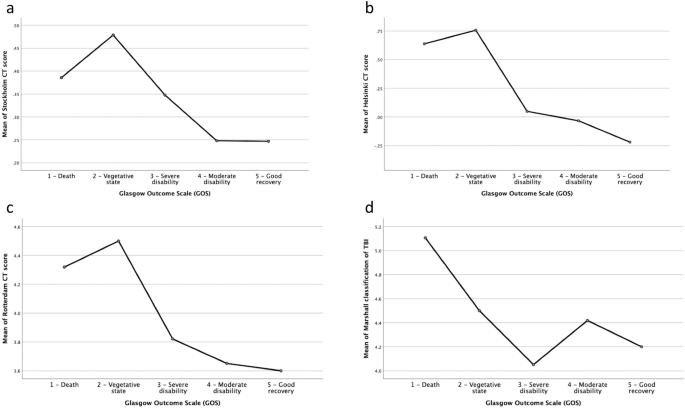
a-d. The relationships between the CT scores, prognostic models and outcome according to GOS

a-b. To illustrate the relationship between IMPACT and CRASH with outcome (GOS), means plots were used where the y-axis shows the percentage of unfavorable outcome and the x-axis shows GOS
The statistical association and accuracy between the different CT-scoring systems and GOS are given in Table 2 , for favorable versus unfavorable outcome, and in Table 3 for dead versus alive. As seen in Table 2 , the Stockholm CT score remained strongest when comparing with other scoring systems, followed by the Helsinki CT score. The Helsinki score was superior in predicting survival (Table 3 ). When adding variables from IMPACT base model to the CT scores, the Stockholm CT score yielded statistical significance in relationship to actual outcome (see Table 4 ).
In this study, we aimed to investigate suitable CT variables and prognostic models to use in clinical practice and research in patients with known outcomes after TBI. There was a significant correlation between the predicted outcome from the scoring systems and the known outcome in the patient series. The Helsinki CT score was most accurate in predicting survival, and the Stockholm CT score was most accurate in predicting unfavorable versus favorable outcome. Furthermore, when the Stockholm CT score was added to the IMPACT base model, this yielded the strongest prognostic relationship compared to any of the other CT scoring systems.
The superior accuracy in the Stockholm and Helsinki CT scores, compared with the Marshall classification and Rotterdam scoring system, might be explained by the inclusion of additional variables, but there were also differences in how the variables were registered and how they affected prognosis. When comparing the Stockholm CT score to the other scores, there was a clear difference in the analyses of traumatic subarachnoid hemorrhage (tSAH). In the Stockholm CT score, absence or presence of intraventricular hemorrhage (IVH) analyzed, as well as the absence or presence of tSAH, both in the convexities and in the basal cisterns, in addition to the volume of the hematoma. The Marshall classification neither included tSAH nor IVH and the Rotterdam score only analyzed the absence or presence of IVH or tSAH, but did not discriminate between the two, and the Helsinki CT score analyzed only the absence or presence of IVH. Considering these factors, it is reasonable to believe that tSAH plays an important role in the prognosis of TBI patients, i.e. a worse outcome in the presence of tSAH, which has also been shown in previous studies [ 21 ]. One reason for this could be more complicated management as traumatic subarachnoid blood might case harmful complications such as vasospasm, ischemia, hydrocephalus, and electrolyte disturbances [ 22 ].
Another difference between the scoring systems concerned epidural hematomas (EDH), where the Helsinki CT score and the Stockholm CT score considered the presence of EDH a positive prognostic factor, and if present, subtracted points for EDH from the total sum; hence, yielding a more favorable outcome [ 19 ]. However, in the Rotterdam scoring system, the presence of EDH was considered a negative sign and increased the risk of poor outcome [ 17 ]. In the Marshall classification, hematomas or contusions greater than 25 cm 3 were considered, but EDH was not specifically analyzed. Furthermore, the Stockholm CT score was the only modality which analyzed midline shift as a continuous variable and was also the only scoring system considering if signs of diffuse axonal injury (DAI) were present on CT. Even though DAI is primarily diagnosed on magnetic resonance imaging (MRI) scans, signs of DAI on CT scans could indicate severe consequences [ 23 , 24 ]. Measuring midline shift as a continuous variable and including signs of DAI might explain why the Stockholm CT score was marginally more accurate than the Helsinki CT score in outcome prediction.
We found that adding relevant CT variables to the IMPACT base model yielded improved prognostic measurements. This motivates further investigations to optimize CT findings to increase prognostic accuracy which, in turn, can aid decision making in the clinical setting. From the results of this investigation, we would suggest the use of the Stockholm CT score or Helsinki CT score as the method of choice when predicting prognosis in patients with TBI. For future perspectives, we expect to see additional and more complex prognostic models in TBI, applying the results from large multi-center investigations such as the CENTER-TBI study that includes CT variables as well as biomarkers and clinical baseline data [ 25 ].
Future prospectives in TBI prognostication
Native CT scan is part of the routine trauma protocol used at patient admission. The prognostic scores investigated in this study use findings on a native CT scan for prognostication. However, data suggests that perfusion CT scans could be superior in prognostication after TBI [ 26 ]. As CT perfusion measures cerebral vascular autoregulation, it could be useful in the guidance of TBI treatment [ 27 , 28 ]. Further studies on TBI prognostication should include CT perfusion and aim to address physiology as well as associated injuries to improve prognosis and overall care for these patients.
Strengths and limitations
The patients in this investigation were highly representative of TBI-patients in NICU. Only a small number of patients were lost to follow-up, and the standards of care adhere to the Brain trauma foundation guidelines [ 29 ] and are also routine practice in other Scandinavian and international level 1 trauma centers and NICU. Data collection was retrieved by a limited number of individuals in the team, and the radiological examinations were performed according to a routine trauma protocol and available for assessment.
One limitation was that the study was retrospective and relied on data from medical records. Moreover, when commencing this study, one aim was to compare the known patient outcome with the predicted outcome from the different prognostic models for each individual. The reason for this was to assess whether the prognostic tools yielded a poorer outcome compared to reality – or in fact a more positive outcome – and in turn, this would be another guide in the clinical setting. However, we could not find a statistical model to analyze this, due to the differences in the nature of the values of GOS and the prognostic tools. Furthermore, we assessed GOS one-year post-injury, and the prognostic models generally present outcomes at 6 months. This was a limitation in the view of comparison, and we suspect that the outcomes in our patients would be better, given the six additional months of recovery. However, the improvement beyond 6 months seems limited in reported literature [ 30 , 31 ].
A possible limitation of the study was that different CT machines and protocols were used during the study period. However, for native CT scans, this has less effect compared to more advanced modalities. Additionally, a Swedish survey from 2016 showed that homogenous examination protocols for CT head scans were used in trauma and 98% used native CT scans without contrast [ 32 ]. All scanners at the different hospitals in the region underwent regular maintenance by the vendors and sequences were optimized by the hospitals for clinical evaluation of traumatic brain injuries.
There was a significant correlation between the predicted outcomes from the scoring systems and the known outcomes in this patient material, representative for a NICU TBI-population. The Stockholm CT score and the Helsinki score yielded the closest relationship between the models and the actual outcomes. Furthermore, the Stockholm CT score yielded the strongest overall relationship when adding variables from IMPACT base model and would be our method of choice for continued research when using any of the current available CT scoring models.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Computed tomography
Diffuse axonal injury
Epidural hematoma
Glasgow coma scale
Glasgow outcome scale
Intraventricular hemorrhage
Magnetic Resonance Imaging
Neurointensive Care Unit
Subarachnoid hemorrhage
Subdural hematoma
- Traumatic brain injury
Traumatic subarachnoid hemorrhage
Faul M, Coronado V. Epidemiology of traumatic brain injury. Handb Clin Neurol. 2015;127:3–13. https://doi.org/10.1016/B978-0-444-52892-6.00001-5 .
Article PubMed Google Scholar
Stalnacke BM, Saveman BI, Stenberg M. Long-term follow-up of disability, cognitive, and emotional impairments after severe traumatic brain injury. Behav Neurol. 2019;2019:9216931.
Article PubMed PubMed Central Google Scholar
Tagliaferri F, Compagnone C, Korsic M, Servadei F, Kraus J. A systematic review of brain injury epidemiology in Europe. Acta Neurochir. 2006;148(3):255–68; discussion 68. https://doi.org/10.1007/s00701-005-0651-y .
Article PubMed CAS Google Scholar
Gao J, Zheng Z. Development of prognostic models for patients with traumatic brain injury: a systematic review. Int J Clin Exp Med. 2015;8(11):19881–5.
PubMed PubMed Central CAS Google Scholar
Czeiter E, Mondello S, Kovacs N, Sandor J, Gabrielli A, Schmid K, et al. Brain injury biomarkers may improve the predictive power of the IMPACT outcome calculator. J Neurotrauma. 2012;29(9):1770–8. https://doi.org/10.1089/neu.2011.2127 .
Perel P, Arango M, Clayton T, Edwards P, Komolafe E, Poccock S, et al. Predicting outcome after traumatic brain injury: practical prognostic models based on large cohort of international patients. BMJ (Clinical research ed). 2008;336(7641):425–9. https://doi.org/10.1136/bmj.39461.643438.25 .
Article Google Scholar
Steyerberg EW, Mushkudiani N, Perel P, Butcher I, Lu J, McHugh GS, et al. Predicting outcome after traumatic brain injury: development and international validation of prognostic scores based on admission characteristics. PLoS Med. 2008;5(8):e165 discussion e.
Brennan PM, Murray GD, Teasdale GM. Simplifying the use of prognostic information in traumatic brain injury. Part 1: the GCS-pupils score: an extended index of clinical severity. J Neurosurg. 2018;128(6):1612–20. https://doi.org/10.3171/2017.12.JNS172780 .
Murray GD, Brennan PM, Teasdale GM. Simplifying the use of prognostic information in traumatic brain injury. Part 2: graphical presentation of probabilities. J Neurosurg. 2018;128(6):1621–34. https://doi.org/10.3171/2017.12.JNS172782 .
Olivecrona M, Koskinen LO. The IMPACT prognosis calculator used in patients with severe traumatic brain injury treated with an ICP-targeted therapy. Acta Neurochir. 2012;154(9):1567–73. https://doi.org/10.1007/s00701-012-1351-z .
Charry JD, Navarro-Parra S, Solano J, Moscote-Salazar L, Pinzón MA, Tejada JH. Outcomes of traumatic brain injury: the prognostic accuracy of various scores and models. Neurol Neurochir Pol. 2019;53(1):55–60. https://doi.org/10.5603/PJNNS.a2018.0003 .
Maeda Y, Ichikawa R, Misawa J, Shibuya A, Hishiki T, Maeda T, et al. External validation of the TRISS, CRASH, and IMPACT prognostic models in severe traumatic brain injury in Japan. PLoS One. 2019;14(8):e0221791. https://doi.org/10.1371/journal.pone.0221791 .
Bilgi K, Gopalakrishna KN, Chakrabarti D, Rao GSU. Outcome prediction of TBI: are there parameters that affect the IMPACT and CRASH models? World neurosurgery; 2020.
Google Scholar
Wongchareon K, Thompson HJ, Mitchell PH, Barber J, Temkin N. IMPACT and CRASH prognostic models for traumatic brain injury: external validation in a south-American cohort. Inj Prev. 2020;26(6):546–54. https://doi.org/10.1136/injuryprev-2019-043466 .
Maas AI, Murray GD, Roozenbeek B, Lingsma HF, Butcher I, McHugh GS, et al. Advancing care for traumatic brain injury: findings from the IMPACT studies and perspectives on future research. The Lancet Neurology. 2013;12(12):1200–10. https://doi.org/10.1016/S1474-4422(13)70234-5 .
Marshall LF, Marshall SB, Klauber MR, Van Berkum CM, Eisenberg H, Jane JA, et al. The diagnosis of head injury requires a classification based on computed axial tomography. J Neurotrauma. 1992;9(Suppl 1):S287–92.
PubMed Google Scholar
Maas AI, Hukkelhoven CW, Marshall LF, Steyerberg EW. Prediction of outcome in traumatic brain injury with computed tomographic characteristics: a comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery. 2005;57(6):1173–82 discussion −82.
Raj R, Siironen J, Skrifvars MB, Hernesniemi J, Kivisaari R. Predicting outcome in traumatic brain injury: development of a novel computerized tomography classification system (Helsinki computerized tomography score). Neurosurgery. 2014;75(6):632–46; discussion 46-7. https://doi.org/10.1227/NEU.0000000000000533 .
Nelson DW, Nyström H, MacCallum RM, Thornquist B, Lilja A, Bellander BM, et al. Extended analysis of early computed tomography scans of traumatic brain injured patients and relations to outcome. J Neurotrauma. 2010;27(1):51–64. https://doi.org/10.1089/neu.2009.0986 .
Wilson JT, Pettigrew LE, Teasdale GM. Structured interviews for the Glasgow outcome scale and the extended Glasgow outcome scale: guidelines for their use. J Neurotrauma. 1998;15(8):573–85. https://doi.org/10.1089/neu.1998.15.573 .
Thelin EP, Nelson DW, Vehviläinen J, Nyström H, Kivisaari R, Siironen J, et al. Evaluation of novel computerized tomography scoring systems in human traumatic brain injury: an observational, multicenter study. PLoS Med. 2017;14(8):e1002368. https://doi.org/10.1371/journal.pmed.1002368 .
Modi NJ, Agrawal M, Sinha VD. Post-traumatic subarachnoid hemorrhage: a review. Neurol India. 2016;64(Suppl):S8–s13.
Vieira RC, Paiva WS, de Oliveira DV, Teixeira MJ, de Andrade AF, de Sousa RM. Diffuse axonal injury: epidemiology, Outcome and Associated Risk Factors. Front Neurol. 2016;7:178.
Ljungqvist J, Nilsson D, Ljungberg M, Esbjörnsson E, Eriksson-Ritzén C, Skoglund T. Longitudinal changes in diffusion tensor imaging parameters of the corpus callosum between 6 and 12 months after diffuse axonal injury. Brain Inj. 2017;31(3):344–50. https://doi.org/10.1080/02699052.2016.1256500 .
Dijkland SA, Retel Helmrich IRA, Nieboer D, van der Jagt M, Dippel DWJ, Menon D, et al. Outcome Prediction after Moderate and Severe Traumatic Brain Injury: External Validation of Two Established Prognostic Models in 1742 European patients. J Neurotrauma. 2020;38(10):1377-88.
Wintermark M, van Melle G, Schnyder P, Revelly JP, Porchet F, Regli L, et al. Admission perfusion CT: prognostic value in patients with severe head trauma. Radiology. 2004;232(1):211–20. https://doi.org/10.1148/radiol.2321030824 .
Wintermark M, Chiolero R, Van Melle G, Revelly JP, Porchet F, Regli L, et al. Cerebral vascular autoregulation assessed by perfusion-CT in severe head trauma patients. J Neuroradiol. 2006;33(1):27–37. https://doi.org/10.1016/S0150-9861(06)77225-X .
Wintermark M, Chioléro R, van Melle G, Revelly JP, Porchet F, Regli L, et al. Relationship between brain perfusion computed tomography variables and cerebral perfusion pressure in severe head trauma patients. Crit Care Med. 2004;32(7):1579–87. https://doi.org/10.1097/01.CCM.0000130171.08842.72 .
Carney N, Totten AM, O'Reilly C, Ullman JS, Hawryluk GW, Bell MJ, et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery. 2017;80(1):6–15. https://doi.org/10.1227/NEU.0000000000001432 .
Pagulayan KF, Temkin NR, Machamer J, Dikmen SS. A longitudinal study of health-related quality of life after traumatic brain injury. Arch Phys Med Rehabil. 2006;87(5):611–8. https://doi.org/10.1016/j.apmr.2006.01.018 .
Stocchetti N, Zanier ER. Chronic impact of traumatic brain injury on outcome and quality of life: a narrative review. Crit Care. 2016;20(1):148. https://doi.org/10.1186/s13054-016-1318-1 .
Wiklund E, Koskinen SK, Linder F, Åslund PE, Eklöf H. Whole body computed tomography for trauma patients in the Nordic countries 2014: survey shows significant differences and a need for common guidelines. Acta Radiol. 2016;57(6):750–7. https://doi.org/10.1177/0284185115597718 .
Download references
Acknowledgments
The authors would like to thank statistician Nashmil Ariai for aiding with the statististical analyses.
AC received financial support from The Gothenburg Society of Medicine. Funding was received from ALF, the Swedish national contract between the state and region in cooperation with the university and the hospital in purpose to fund education and clinical research to advance development in medicine and health care. Open Access funding provided by University of Gothenburg.
Author information
Authors and affiliations.
Department of Neurosurgery, Sahlgrenska University Hospital, SE-413 45, Gothenburg, Sweden
Djino Khaki, Alba Corell & Johan Ljungqvist
Department of Clinical Neuroscience, Institute of Neuroscience and Physiology, The Sahlgrenska Academy at the University of Gothenburg, Gothenburg, Sweden
Department of Anesthesia and Intensive Care, Sahlgrenska University Hospital, Gothenburg, Sweden
Virpi Hietanen & Helena Odenstedt Hergès
Institute of Anesthesiology and Intensive Care Medicine, The Sahlgrenska Academy at the University of Gothenburg, Gothenburg, Sweden
Helena Odenstedt Hergès
You can also search for this author in PubMed Google Scholar
Contributions
JL, VH and DK were involved in the planning of the study and study design. DK, VH and JL performed the data collection. DK, JL and AC performed the manuscript writing and submission process. HOH and VH drafted the manuscript. All authors substantively revised the manuscript and has approved the submitted version.
Corresponding authors
Correspondence to Djino Khaki or Johan Ljungqvist .
Ethics declarations
Ethics approval and consent to participate, consent for publication.
Not applicable.
Competing interests
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: supplementary table 1..
Overview of the different prognostic calculators and CT scores.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Khaki, D., Hietanen, V., Corell, A. et al. Selection of CT variables and prognostic models for outcome prediction in patients with traumatic brain injury. Scand J Trauma Resusc Emerg Med 29 , 94 (2021). https://doi.org/10.1186/s13049-021-00901-6
Download citation
Received : 12 April 2021
Accepted : 11 June 2021
Published : 17 July 2021
DOI : https://doi.org/10.1186/s13049-021-00901-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- IMPACT score
- CRASH score
- Stockholm CT score
- Marshall classification
- Rotterdam scoring system
- Helsinki CT score
Scandinavian Journal of Trauma, Resuscitation and Emergency Medicine
ISSN: 1757-7241
- Submission enquiries: [email protected]
- General enquiries: [email protected]
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List
Predicting outcome after traumatic brain injury: practical prognostic models based on large cohort of international patients
- Author information
- Article notes
- Copyright and License information
Correspondence to: P A Perel [email protected]
Accepted 2007 Dec 5; Issue date 2008 Feb 23.
Objective To develop and validate practical prognostic models for death at 14 days and for death or severe disability six months after traumatic brain injury.
Design Multivariable logistic regression to select variables that were independently associated with two patient outcomes. Two models designed: “basic” model (demographic and clinical variables only) and “CT” model (basic model plus results of computed tomography). The models were subsequently developed for high and low-middle income countries separately.
Setting Medical Research Council (MRC) CRASH Trial.
Subjects 10 008 patients with traumatic brain injury. Models externally validated in a cohort of 8509.
Results The basic model included four predictors: age, Glasgow coma scale, pupil reactivity, and the presence of major extracranial injury. The CT model also included the presence of petechial haemorrhages, obliteration of the third ventricle or basal cisterns, subarachnoid bleeding, midline shift, and non-evacuated haematoma. In the derivation sample the models showed excellent discrimination (C statistic above 0.80). The models showed good calibration graphically. The Hosmer-Lemeshow test also indicated good calibration, except for the CT model in low-middle income countries. External validation for unfavourable outcome at six months in high income countries showed that basic and CT models had good discrimination (C statistic 0.77 for both models) but poorer calibration.
Conclusion Simple prognostic models can be used to obtain valid predictions of relevant outcomes in patients with traumatic brain injury.
Introduction
Traumatic brain injury is a leading cause of death and disability worldwide. Every year, about 1.5 million affected people die and several millions receive emergency treatment. 1 2 Most of the burden (90%) is in low and middle income countries. 3
Clinicians treating patients often make therapeutic decisions based on their assessment of prognosis. According to a 2005 survey, 80% of doctors believed that an accurate assessment of prognosis was important when they made decisions about the use of specific methods of treatment such as hyperventilation, barbiturates, or mannitol. 4 A similar proportion considered that this was important in deciding whether or not to withdraw treatment. Assessment of prognosis was also deemed important for counselling patients and relatives. Only a third of doctors, however, thought that they accurately assessed prognosis. 4
Prognostic models are statistical models that combine data from patients to predict outcome and are likely to be more accurate than simple clinical predictions. 5 The use of computer based prediction of outcome in patients with traumatic brain injury increases the use of certain therapeutic interventions in those predicted to have a good outcome and reduces their use in those predicted to have a poor outcome. 6
Many prognostic models have been reported but none are widely used. A recent systematic review offers possible explanations. 7 Most models were developed on small samples, most were methodologically flawed, and few were validated in external populations. Few were presented in a clinically practical way, nor were they developed in populations from low and middle income countries, where most trauma occurs.
The Medical Research Council (MRC) CRASH (corticosteroid randomisation after significant head injury) trial is the largest clinical trial conducted in patients with traumatic brain injury and presents a unique opportunity to develop a prognostic model. 8 9 The trial prospectively included patients within eight hours of the injury, used standardised definitions of variables, and achieved almost complete follow-up at six months. Furthermore, the large sample size guarantees precise and valid predictions. The high recruitment of patients from low and middle income countries means that models developed with these data are relevant to these settings.
We have developed and validated prognostic models for death at 14 days and death and disability at six months in patients with traumatic brain injury.
Patients —The study cohort was all 10 008 patients enrolled in the trial. Adults with traumatic brain injury, who had a score on the Glasgow coma scale of 14 or less, and who were within eight hours of injury, were eligible for inclusion in the trial.
Outcomes —Death of a patient was recorded on an early outcome form that was completed at hospital discharge, death, or 14 days after randomisation (whichever occurred first). Unfavourable outcome (death or severe disability) at six months was defined with the Glasgow outcome scale (see box). The scale comprises five categories: death, vegetative state, severe disability, moderate disability, and good recovery. For the purpose of this analysis, we dichotomised outcomes into favourable (moderate disability or good recovery) and unfavourable (dead, vegetative state, or severe disability). 10
Category and definition on Glasgow outcome scale
Good recovery: able to return to work or school
Moderate disability: able to live independently; unable to return to work or school
Severe disability: able to follow commands/unable to live independently
Persistent vegetative state: unable to interact with environment; unresponsive
Prognostic variables —For the prognostic model we considered age, sex, cause of injury, time from injury to randomisation, Glasgow coma score at randomisation, pupil reactivity, results of computed tomography, whether the patient had sustained a major extracranial injury, and level of income in country (high or low-middle income countries, as defined by the World Bank) (see table A on bmj.com). 11 We adjusted analyses for treatment within the trial as this was related to outcome, and we did not find interaction between treatment and the potential predictors. 8 9
Analysis —Most of the variables collected in the CRASH trial have been previously associated with prognosis in traumatic brain injury, so we included all of them in a first multivariable logistic regression analysis. 12 We excluded variables that were not significant at 5% level. We quantified each variable’s predictive contribution by its z score (the model coefficient divided by its standard error). We explored linearity between age and mortality at 14 days and Glasgow coma score and mortality at 14 days. Interactions between country income level and all the other predictors were evaluated with a likelihood ratio test. Because there were few data missing, we performed a complete case analysis.
Prognostic models —We developed different models for each of the two outcomes: a basic model, which included only clinical and demographic variables, and a CT model, which also included results of computed tomography.
Performance of the model —We assessed performance of the models in terms of calibration and discrimination. Calibration was assessed graphically and with the Hosmer-Lemeshow test. Discrimination was assessed with the C statistic (an equivalent concept to area under the receiver operator characteristic curve). 13
Internal validation —The internal validity of the final model was assessed by the bootstrap re-sampling technique. Regression models were estimated in 100 models. For each of 100 bootstrap samples we refitted and tested the model on the original sample to obtain an estimate of predictive accuracy corrected for bias. This showed no overoptimism in any of the final model’s predictive C statistics.
External validation —A good prognostic model should be generalisable to populations different to those in which it was derived. 14 We externally validated the models in an external cohort of 8509 patients with moderate and severe traumatic brain injury from 11 studies conducted in high income countries (the IMPACT (international mission for prognosis and clinical trial) dataset). 15
Score development —We developed a clinical score based on regression coefficients. A web based version of the model was developed to be accessible to clinicians internationally.
General characteristics
Table 1 shows the characteristics of the patients. More of the patients were men (81%) and more came from low-middle income countries (75%). More than half (58%) of participants were included within three hours of injury. Road traffic crashes were the most common cause of injury (65%) and 79% of the participants underwent computed tomography. A total of 1948 patients (19%) died in the first two weeks, 2323 patients (24%) were dead at six months, and 3556 patients (37%) were dead or severely dependent at six months.
General characteristics of study population
*P value for comparison between low-middle income countries and high income countries.
The relation between age and the log odds of death within 14 days showed no association until the age of 40 and a linear increase afterwards. The relation between Glasgow coma score and mortality at 14 days was reasonably linear and we therefore included the coma score as a continuous variable (figs 1 and 2) . The relation with unfavourable outcome at six months showed similar patterns.
Fig 1 Relation between age and mortality at 14 days
Fig 2 Relation between Glasgow coma scale and mortality at 14 days
Low-middle v high income countries
In comparison with patients from high income countries, those from low-middle income countries were younger, more likely to be male, were recruited later, had less severe traumatic brain injury (as defined by Glasgow coma score and pupil reactivity), and more often had abnormal results on computed tomography. Road traffic crashes were a more common cause of traumatic brain injury. Although patients from low-middle income countries experienced higher mortality at 14 days (odds ratio 1.94, 95% confidence interval 1.64 to 2.30), there was no significant difference in unfavourable outcome at six months.
There were significant interactions between the country’s income level and several predictors and so we developed two models, one for low-middle income countries and another for high income countries. Older age was a stronger predictor of 14 day mortality in high income countries (interaction P<0.001), and lower Glasgow coma score was a stronger predictor in low-middle income countries (interaction P=0.003). Obliteration of the third ventricle and a non-evacuated haematoma were both associated with a higher risk in high income countries (interaction P<0.001 and P=0.03, respectively).
Multivariable predictive models
We developed eight models altogether: basic and CT models for predicting two outcomes in two settings (low-middle and high income countries).
Basic models —We included four predictors in the basic model: age, Glasgow coma score, pupil reactivity, and the presence of major extracranial injury (table 2). Glasgow coma score was the strongest predictor of outcome in low-middle income countries and age was the strongest predictor in high income countries, while the absence of pupil reactivity was the third strongest predictor in both regions.
Multivariable basic predictive models (excluding data from computed tomography*). Figures are odds ratios (95% confidence intervals) with z scores
GCS=Glasgow coma scale.
*Includes age, GCS, sex, hours since injury, cause of injury, pupil reactivity, and presence of major extracranial injury.
†Per 10 year increase after 40 years.
‡Per decrease of each value of GCS.
CT models —The following characteristics on computed tomography were strongly associated with the outcomes in addition to the predictors included in the basic models: presence of petechial haemorrhages, obliteration of the third ventricle or basal cisterns, subarachnoid bleeding, midline shift, and non-evacuated haematoma (table 3). Obliteration of the third ventricle and midline shift were the strongest predictors of mortality at 14 days, and non-evacuated haematoma was the strongest predictor of unfavourable outcome at six months.
Multivariable predictive models with computed tomography*. Figures are odds ratios (95% confidence intervals) with z scores
*Includes age, GCS, pupil reactivity, presence of major extra cranial injury, and all findings on computed tomography.
Performance of models —All models showed excellent discrimination, with C statistics over 0.80 (tables 2 and 3). Calibration in all models was adequate and six out of the eight models had good calibration when evaluated with the Hosmer-Lemeshow test (figs 3 and 4) .
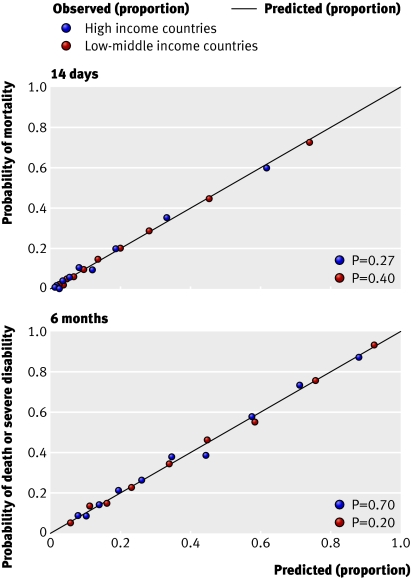
Fig 3 Calibration of basic models using expected and observed probabilities of mortality at 14 days (top) and death or severe disability at six months (bottom) in patient with traumatic brain injury according to income level of country. P value is for Hosmer-Lemeshow test
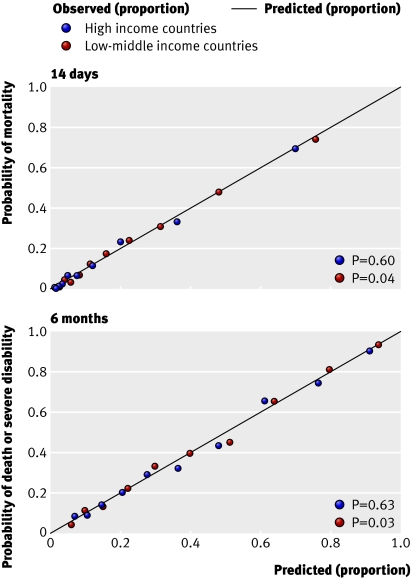
Fig 4 Calibration of computed tomography models using expected and observed probabilities of mortality at 14 days (top) and death or severe disability at six months (bottom) in patient with traumatic brain injury according to income level of country. P value is for Hosmer-Lemeshow test
Clinical score —Individual scores and their respective probability of outcome can be obtained from our web based calculator ( www.crash2.lshtm.ac.uk/ ). By entering the values of the predictors, we can obtain the expected risk of death at 14 days and of death or severe disability at six months. Figure 5 shows a sample screenshot of the predictions for a 26 year old patient from a low and middle income country (Argentina), with a Glasgow coma score of 11, one pupil reactive, and absence of a major extra cranial injury. According to the basic model this patient has a probability of death at 14 days of 10% and a 23.9% risk of death or severe disability at six months. A good agreement is evident between observed and predicted outcome by the web calculator (figs 3 and 4).
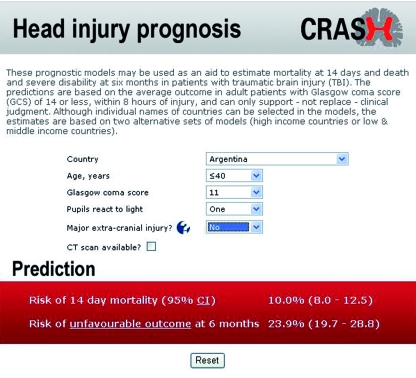
Fig 5 Screenshot of web based calculator available at www.crash2.lshtm.ac.uk/ . If CT scan available box is ticked, calculator displays additional CT variables
External validation —Because an external cohort of patients from low-middle income countries was not available, we validated the models in patients from high income countries only. The IMPACT dataset used for the validation did not include mortality at 14 days and so we could validate only models for unfavourable outcome at six months. We validated the basic model with the variables age, Glasgow coma score, and pupil reactivity. We did not include the variable “major extracranial injury” as it was not available in the validation sample. For the CT models, we added obliteration of the third ventricle or basal cisterns, subarachnoid bleeding, midline shift, and non-evacuated haematoma to the basic model. Similarly, we excluded the variable “petechial haemorrhages” as this was not available in the validation sample. For the validation process we first ran these models in the CRASH trial cohort and we then applied the corresponding coefficients in the validation sample. Although discrimination was, as expected, lower than in the original data, it was still quite good for both the basic and CT models (C statistic 0.77 for both models). The calibration was excellent for the CT model but poorer for the basic model (figs 6 and 7).
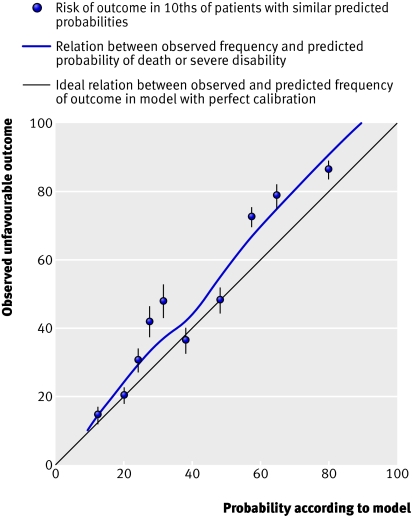
Fig 6 External validation of basic model for death or severe disability at six months in IMPACT database
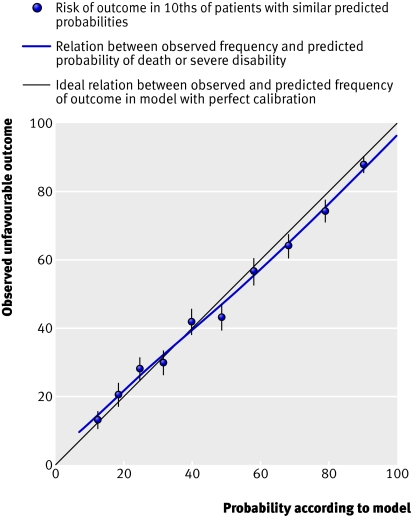
Fig 7 External validation of CT model for death or severe disability at six months in IMPACT database
We have developed web based prognostic models for predicting two clinically relevant outcomes in patients with traumatic brain injury using variables that are available at the bedside. The models have excellent discrimination and good fit with both internal and external validation. We have reported on differences in outcomes and on the strength of predictors of outcomes, according to whether patients are from high or low-middle income countries.
Older age, low Glasgow coma score, absent pupil reactivity, and the presence of major extracranial injury predict poor prognosis. All of these variables have been previously identified as prognostic factors for poor outcome in traumatic brain injury. 12 Glasgow coma score showed a clear linear relation with mortality. Our finding that mortality in patients with Glasgow coma score of 3 was lower than in patients with a score of 4 may be because scores of sedated patients are reported as 3. Increasing age was associated with worse outcomes but this association was apparent only after age 40. A similar threshold has been reported elsewhere. 16 17 Plausible explanations for this include extracranial comorbidities, changes in brain plasticity, or differences in clinical management associated with increasing age. The presence of “obliteration of third ventricle or basal cisterns” on computed tomography was associated with the worst prognosis at 14 days. This is supported by recent findings that absence of basal cisterns is the strongest predictor of six month mortality. 18 We also found—as previously reported—the independent prognostic value of traumatic subarachnoid haemorrhage. 19
Patients from low-middle income countries had worse early prognosis than those from high income countries. Regional differences in outcome after traumatic brain injury have previously been reported between Europe and North America, but the difference in mortality between low-middle and high income countries has not been explored. 20
The strength of association between some predictors and outcomes differed by region. A low Glasgow coma score had an even worse prognosis in patients from low-middle income countries compared with patients from high income countries. This might relate to quality of care or it could be that low Glasgow coma score in high income countries is associated with greater use of sedation, rather than to severity of traumatic brain injury. Increasing age had a worse prognosis in high income countries compared with low-middle income countries. This is because of even lower risks at younger ages in high income countries, while both have similar risks at older ages. Regarding computed tomography, some abnormal findings were stronger predictors in high income countries. This could be because of better technology and therefore more accurate diagnosis with computed tomography.
A systematic review identified over 100 prognostic models for patients with traumatic brain injury, but methodological quality was adequate in only a few. 7 As with our models, two of the more methodologically robust models showed similarly good discrimination but worse calibration. 17 21 They too included Glasgow coma score, age, pupil reactivity, and results of computed tomography as predictors, but, unlike our models, they did not include the presence of major extracranial injury, and none of them included patients from low-middle income countries.
Strengths and weakness of the study
Our study’s strengths are the use of a well described cohort of patients, prospective and standardised collection of data on prognostic factors, low loss to follow-up, and the use of a validated outcome measure at a fixed time after the injury. All of these factors provide reassurance about the internal validity of our models. The large sample size in relation to the number of prognostic variables examined is another particular strength. In relation to its external validity, only a few prognostic models have been developed from patients in low-middle income countries, and to the best of our knowledge the models we developed are the first with a large sample size and adequate methods. 7 The external validation confirmed the discriminatory ability of the models in patients from high income countries and showed good calibration for the computed tomography model. Unlike most published prognostic models, we included the complete spectrum of patients with traumatic brain injury, ranging from mild to severe. Finally, the data required to make predictions with the model are easily available to clinicians, and we have developed a web based risk calculator.
There are some limitations. The data from which the models were developed come from a clinical trial and this could therefore limit external validity. For example, patients were recruited within eight hours of injury and we cannot estimate the accuracy of the models for patients evaluated beyond this time. Nevertheless, the CRASH trial was a pragmatic trial that did not require any additional tests and therefore included a diversity of “real life” patients. Another limitation was that for the validation we were forced to exclude the variables major extracranial injury and petechial haemorrhages because they were not available in the IMPACT sample. Neither of these variables, however, was among the stronger predictors. The external validation showed good discriminatory ability, but this was somewhat lower than in the original data. This may be explained by a more homogeneous selected case mix in these other trials, which included only patients with moderate and severe Glasgow coma score.
Implications of the study
Most of the burden of traumatic brain injury is in low-middle income countries, where case fatality is higher and resources are limited. We found that several predictors differed in their strength of association with outcome according to income level of country, suggesting that it may be inappropriate to extrapolate from models for high income countries to poorer settings. We have developed a methodologically valid, simple, and accurate model that may help decisions about health care for individual patients. It is important to emphasise, however, that while prognostic models may complement clinical decision making they cannot and should not replace clinical judgment. This is particularly important in the context of judgments about the withdrawal of care or clinical triage. These prognostic models can also help in the design and analysis of clinical trials, through prognostic stratification, and can be used in clinical audit by allowing adjustment for case mix. 22
Future research
The differences found between the prognostic models for low-middle and high income countries are important. Although most of the burden of trauma occurs in low-middle income countries, most research takes place in high income countries. 3 A recent systematic review found that few prognostic models for traumatic brain injury were developed in low-middle income countries. 7 More research is therefore needed to obtain reliable data from these settings. An improved understanding of the differences between these regions might also clarify the mechanisms of predictors that are not immediately obvious when we analyse a homogeneous population. Because our models were developed with data from a clinical trial, and validated only in patients from high income countries, further prospective validation in independent cohorts is needed to strengthen the generalisability of the models. Future research could also evaluate different ways, or formats, for presenting the models to physicians; their use in clinical practice; and whether ultimately they have any impact on the management and outcomes of patients with traumatic brain injury.
What is already known on this topic
Traumatic brain injury is a leading cause of death and disability worldwide with most cases occurring in low-middle income countries
Prognostic models may improve predictions of outcome and help in clinical research
Many prognostic models have been published but methodological quality is generally poor, sample sizes small, and only a few models have included patients from low-middle income countries
What this study adds
In a basic model prognostic indicators included age, Glasgow coma scale, pupil reactivity, and the presence of major extracranial injury
In a CT model additional indicators were the presence of petechial haemorrhages, obliteration of the third ventricle or basal cisterns, subarachnoid bleeding, mid-line shift, and non-evacuated haematoma
The strength of predictors of outcomes varies according to whether patients are from high or low-middle income countries
These prognostic models, that include simple variables, are available on the internet ( www.crash2.lshtm.ac.uk/ )
Supplementary Material
CRASH trial collaborators by country (number of patients randomised)
Albania (41 patients): Fatos Olldashi (national coordinator), Itan Muzha (Central Military University Hospital National Trauma Centre, 35); Nikolin Filipi ( University Hospital “Mother Teresa” Tirana, 6).
Argentina (359 patients): Roberto Lede, Pablo Copertari, Carolina Traverso, Alejandro Copertari (IAMBE—national and regional coordinators for southern Latin America); Enrique Alfredo Vergara, Carolina Montenegro, Roberto Ruiz de Huidobro, Pantaleón Saladino (Hospital San Bernardo, 106); Karina Surt, José Cialzeta, Silvio Lazzeri (Hospital Escuela Jose de San Martin, 52); Gustavo Piñero, Fabiana Ciccioli (Hospital Municipal “Dr Leonidas Lucero,” 37); Walter Videtta, María Fernanda Barboza (Hospital Dr Ramón Carrillo, 35); Silvana Svampa, Victor Sciuto (Hospital Castro Rendon, 28); Gustavo Domeniconi, Marcelo Bustamante (Hospital Zonal General De Agudos “Heroes de Malvinas,” 27); Maximiliano Waschbusch (Policlinico Sofia T de Santamarina. 20); María Paula Gullo (Hospital Municipal Dr Hector J D’Agnillo, 17); Daniel Alberto Drago (Hospital Nacional Profesor Alejandro Posadas, 11); Juan Carlos Arjona Linares (Hospital Español de Mendoza, 10); Luis Camputaro (Hospital Italiano, 10); Gustavo Tróccoli (Hospital “Dr José Penna,” 5); Hernán Galimberti (Hospital Aleman, 1).
Australia (13 patients): Mandy Tallott (Gold Coast Hospital, 13).
Austria (21 patients): Christian Eybner, Walter Buchinger (Waldviertelklinikum Standort Horn, 17); Sylvia Fitzal (Wilhelminenspital der Stadt Wien, 4).
Belgium (403 patients): Guy Mazairac (national coordinator), Véronique Oleffe, Thierry Grollinger, Philippe Delvaux, Laurent Carlier (Centre Hospitalier Regional de Namur, 356); Veronique Braet (AZ Klina Hospital, 34); Jean-Marie Jacques (Hospital of Jolimont, 11); Danielle de Knoop (Clinique Saint-Luc, 2).
Brazil (119 patients): Luiz Nasi (national coordinator), Humberto Kukhuyn Choi, Mara Schmitt (Hospital de Pronto Socorro de Porto Alegre, 113); André Gentil (Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, 5); Flavio Nacul (Clínica São Vicente, 1).
Chile (3 patients): Pedro Bedoya Barrios (Hospital Regional Copiapo, 3).
China (87 patients): Chen Xinkang, Lin Shao Hua, Huang Han Tian (Zhongshan City People’s Hospital, 79); Cai Xiaodong (Sheng Zheng Second People’s Hospital, 8).
Colombia (832 patients): Wilson Gualteros, Alvaro Ardila Otero (Hospital Universitario San Jorge, 216); Miguel Arango (national coordinator, regional coordinator for northern Latin America and Caribbean), Juan Ciro, Hector Jaramillo (Gloria GarciaClinica Las Americas, 199); Ignacio Gonzalez, Carolina Gomez (Hospital General de Medellin, 119); Arturo Arias, Marco Fonseca, Carlos Mora (Hospital Erasmo Meoz, 90); Edgar Giovanni Luna Cabrera, José Luis Betancurth, Porfirio Muñoz (Hospital Departamental de Nariño, 51); Jesus Alberto Quiñónez, Maria Esther Gonzalez Castillo (Hospital San Andres, 37); Orlando Lopez (Hospital Federico Lleras, 31); Rafael Perez Yepes, Diana Leon Cuellar, Gerson Paez (Hospital El Tunal, 24); Hernán Delgado Chaves, Pablo Emilio Ordoñez (Hospital Civil de Ipiales, 21); Ricardo Plata, Martha Pineda (Hospital Universitario del Valle, 15); Libardo Enrique Pulido (Hospital Regional de Duitama, 12); John Sergio Velez Jaramillo (Hospital Timothy Britton, 12); Carlos Rebolledo (Organización Clinica General del Norte, 5).
Costa Rica (20 patients): Oscar Palma (Hospital México, 20).
Cuba (404 patients): Caridad Soler (national coordinator); Irene Pastrana, Raul Falero (Hospital Abel Santamaria Cuadrado, 77); Mario Domínguez Perera, Agustín Arocha García, Raydel Oliva (Hospital Universitario “Arnaldo Milián Castro,” 55); Hubiel López Delgado (Hospital Provincial Docente “Manuel Ascunce Domenech,” 43); Aida Madrazo Carnero, Boris Leyva López (Hospital VI Lenin, 42); Angel Lacerda Gallardo, Amarilys Ortega Morales (Hospital General de Morón, 40); Humberto Lezcano (Hospital General Universitario “Carlos Manuel de Cèspedes,” 38); Marcos Iraola Ferrer (Hospital Universitario “Dr Gustavo Aldereguia Lima,” 37); Irene Zamalea Bess, Gladys Rivas Canino (Hospital Miguel Enriquez, 36); Ernesto Miguel Piferrer Ruiz (Hospital Clínico-Quirúrgico Docente “Saturnino Lora,” 32); Orlando Garcia Cruz (Centro de Investigaciones Médico-Quirúrgicas, 4).
Czech Republic (961 patients): Petr Svoboda (national coordinator), Ilona Kantorová, Jiřĺ Ochmann, Peter Scheer, Ladislav Kozumplík, Jitka Maršová (Research Institute for Special Surgery and Trauma, 852); Karel Edelmann (Masaryk Hospital, 41); Ivan Chytra, Roman Bosman (Charles University Hospital, Plzen, 35); Hana Andrejsová (University Hospital Hradec Kralove, 15); Jan Pachl (Hospital Kralovske Vinohgrady, 9); Jan Bürger (Hospital Pribram, 7); Filip Kramar (Univerzity Karlovy Neurochirurgicka Klinika, 2).
Ecuador (258 patients): Mario Izurieta Ulloa (national coordinator), Luis Gonzalez, Alberto Daccach, Antonio Ortega, Stenio Cevallos (Hospital Luis Vernaza, 202); Boris Zurita Cueva (Hospital de la Policia Guayaquil, 16); Marcelo Ochoa (Hospital Jose Carrasco Arteaga, 11); Jaime Velásquez Tapia (Hospital Naval, 11); Jimmy Hurtado (Clínica Central, 8); Miguel Chung Sang Wong (Hospital Militar de Guayaquil, 5); Roberto Santos (Hospital Regional del IESS “Dr Teodoro Maldonado Carbo,” 5).
Egypt (775 patients): Hussein Khamis (national coordinator), Abdul Hamid Abaza, Abdalla Fekry, Salah El Kordy, Tarek Shawky (Mataria Teaching Hospital, 364); Hesham El-Sayed (national coordinator), Nabil Khalil, Nader Negm, Salem Fisal (Suez Canal University, 180); Mamdouh Alamin, Hany Shokry (Aswan Teaching Hospital, 160); Ahmed Yahia Elhusseny, Atif Radwan, Magdi Rashid (Zagazig University Hospital, 71).
Georgia (56 patients): Tamar Gogichaisvili (national coordinator), George Ingorokva, Nikoloz Gongadze (Neurosurgery Department of Tbilisi State Medical University, 55); Alexander Otarashvili (Tbilisi 4th Hospital, 1).
Germany (27 patients): Waltraud Kleist (Ernst Moritz Arndt University, 14); Mathias Kalkum (Kreiskrankenhaus Tirschenreuth, 8); Peter Ulrich (Klinikum Offenbach, 5).
Ghana (7 patients): Nii Andrews (Narh-Bita Hospital, 7).
Greece (20 patients): George Nakos (University Hospital of Ioannina, 8); Antonios Karavelis (University General Hospital of Larissa, 5); George Archontakis (Chania General Hospital “St George,” 4); Pavlos Myrianthefs (KAT Hospital of Athens, 3).
India (973 patients): Yadram Yadav, Sharda Yadav, R Khatri, Arvind Baghel (NSCB Medical College, 177); Mazhar Husain (national coordinator for north India), Deepak Jha (King George Medical College, 105); Wu Hoong Chhang, Manohar Dhandhania, Choden Fonning (North Bengal Neuro Research Centre, 65); S N Iyengar, Sanjay Gupta (G R Medical College, 51); R R Ravi, K S Bopiah, Ajay Herur (Medical Trust Hospital Kochi, 51); N K Venkataramana (national coordinator for south India), A Satish (Manipal Hospital, 50); K Bhavadasan, Raymond Morris, Ramesh S (Medical College Hospital Trivandrum, 50); A Satish (Abhaya Hospital, 42); Yashbir Dewan, Yashpal Singh (Christian Medical College, 36); Rajesh Bhagchandani, Sanjana Bhagchandani (Apex Hospital Bhopal, 32); Vijaya Ushanath Sethurayar (Meenakshi Mission Hospital and Research Centre, 32); Sojan Ipe, G Sreekumar (MOSC Medical College Hospital, 32); Manas Panigrahi, Agasti Reddy (Nizam’s Institute of Medical Sciences, 28); Varinder Khosla, Sunil Gupta (Postgraduate Institute of Medical Education and Research, 28); Haroon Pillay, Nisha Thomas (Baby Memorial Hospital, 25); Krishnamurthy Sridhar, Bobby Jose (V H S Hospital, 22); Nadakkavvkakan Kurian (Jubilee Mission Hospital, 20); Shanti Praharaj, Shibu Pillai (National Institute of Mental Health and Neurosciences, 17); Ramana (Care Hospital (16); Sanjay Gupta, Smita Gupta (Sri Sai Hospital, 16); Dilip Kiyawat (Hirabi Cowasji Jehangir Medical Research Institute, 15); Kishor Maheshwari (Maheshwari Orthopaedic Hospital, 13); Dilip Panikar (Amrita Institute of Medical Sciences, 11); Jayant Chawla (Hartej Maternity and Nursing Home, 7); Satyanarayana Shenoy, Annaswamy Raja (Kasturba Medical College and Hospital, 7); Yeshomati Rupayana (Choitram Hospital and Research Centre, 6); Suryanarayan Reddy (Gowri Gopal Superspeciality Hospital, 6); Nelanuthala Mohan (Apex Hospital Visakhapatnam, 3); Shailesh Kelkar (Central India Institute of Medical Sciences, 3); Yadram Yadav (Marble City Hospital and Research Centre, 3); Jayant Chawla (Government Medical College Amritsar, 1); Mukesh Johri (Johri Hospital, 1); Yadram Yadav (National Hospital Jabalpur, 1).
Indonesia (238 patients): Nyoman Golden (national coordinator), Sri Maliawan (Sanglah General Hospital, 222); Achmad Fauzi, Umar Farouk (Sidoarjo General Hospital, 14).
Iran (233 patients): Esmaeel Fakharian, Amir Aramesh (Naghavi University Hospital, 110); Maasoumeh Eghtedari, Farhad Ahmadzadeh, Alireza Gholami (Fatemeh Zahra Hospital, 85); Maasoumeh Eghtedari, Farhad Ahmadzadeh (Social Security Hospital, 38).
Ireland (113 patients): Patrick Plunkett, Catherine Redican, Geraldine McMahon (St James’s Hospital, 113).
Italy (9 patients): Maria Giuseppina Annetta (Università Cattolica del Sacro Cuore, 4); Homère Mouchaty (Università di Firenze, 3); Eros Bruzzone (Ospedale San Martino, 2).
Ivory Coast (3 patients): Béatrice Harding (CHU de Cocody, 3).
Kenya (2 patients): Mahmood Qureshi (Aga Khan Hospital, 2).
Malaysia (176 patients): Zamzuri Idris, Jafri Abdullah NC, Ghazaime Ghazali, Abdul Rahman Izaini Ghani (Hospital University Science Malaysia, 162); Fadzli Cheah (Ipoh Specialist Hospital, 14).
Mexico (17 patients): Alfredo Cabrera (national coordinator); José Luis Mejía González (Hospital General Regional No 1, 12); José Luis Mejía González (Hospital General de Queretaro, 4); Jorge Loría-Castellanos (Hospital General Regional No 25, 1).
New Zealand (43 patients): Suzanne Jackson, Robyn Hutchinson (Dunedin Hospital, 43).
Nigeria (180 patients): Edward Komolafe (national coordinator), Augustine Adeolu, Morenikeji Komolafe (Obafemi Awolowo University Teaching Hospitals, 77); Olusanya Adeyemi-Doro, Femi Bankole (Lagos University Teaching Hospital, 43); Bello Shehu, Victoria Danlami (Usmanu Danfodiyo University Teaching Hospital, 36); Olugbenga Odebode (University of Ilorin Teaching Hospital, 15); Kehinde Oluwadiya (Lautech Teaching Hospital, 7); Ahmed Sanni (Lagos State University Teaching Hospital, 1); Herb Giebel (Seventh Day Adventist Hospital, 1); Sushil Kumar (St Stephen’s Hospital, 1).
Pakistan (17 patients): Rashid Jooma (Jinnah Postgraduate Medical Centre, 17).
Panama (7 patients): Jose Edmundo Mezquita (Complejo Hospitalario M A Guerrero, 7).
Paraguay (10 patients): Carlos Ortiz Ovelar (Instituto de Prevision Social, 10).
Peru (8 patients): Marco Gonzales Portillo (Hospital Nacional “Dos de Mayo,” 6); Diana Rodriguez (national coordinator) (Hospital Nacional Arzobispo Loayza, 2).
Romania (319 patients): Laura Balica (national coordinator), Bogdan Oprita, Mircea Sklerniacof, Luiza Steflea, Laura Bandut (Spitalul Clinic de Urgenötùa Bucuresö ti, 282); Adam Danil, Remus Iliescu (Sfantum Pantelimon Hospital, 28); Jean Ciurea (Prof Dr D Bagdasar Clinical Emergency Hospital, 9).
Saudi Arabia (32 patients): Abdelazeem El-Dawlatly, Sherif Alwatidy (King Khalid University Hospital, 24); Walid Al-Yafi, Megahid El-Dawlatly (King Khalid National Guard Hospital, 8).
Serbia (23 patients): Ranka Krunic-Protic, Vesna Janosevic (Klinicki Centar Srbije, 23).
Singapore (23 patients): James Tan (national coordinator) (National Neuroscience Institute, 21); Charles Seah (Changi General Hospital, 2).
Slovakia (179 patients): Štefan Trenkler (national coordinator), Matuš Humenansky, Tatiana Stajančová (Reiman Hospital, 71); Ivan Schwendt, Anton Laincz (NsP Poprad, 39); Zeman Julius, Stano Maros (Nemocnica Bojnice, 25); Jozef Firment (FNsp Kosice, 12); Maria Cifraničova (NsP Trebisov, 11); Beata Sániová (Faculty Hospital in Martin, 10); Karol Kalig (NsP Ruzinov, 4); Soňa Medekova (NsP Nové Zámky, 3); Radovan Wiszt (NsP Liptovsky Mikulas, 2); NsP F D Roosevelt, 1); Ivan Mačuga (NsP Zilina, 1).
South Africa (366 patients): Bennie Hartzenberg (national coordinator), Grant du Plessis, Zelda Houlie (Tygerberg Academic Hospital, 307); Narendra Nathoo, Sipho Khumalo (Wentworth Hospital, 57); Ralph Tracey (Curamed Kloof Hospital, 1).
Spain (259 patients): Angeles Muñoz-Sánchez (national coordinator), Francisco Murillo-Cabezas NC, Juan Flores-Cordero, Dolores Rincón-Ferrari (Hospital Universitario Virgen del Rocio, 133); Martin Rubi, Lopez Caler (Hospital Torrecárdenas, 37); Maite Misis del Campo, Luisa Bordejé Laguna (Hospital Universitario Germans Trias i Pujol, 32); Juan Manuel Nava (Hospital Mútua de Terrassa, 20); Miguel Arruego Minguillón (Hospital Universitario de Girona Dr Josep Trueta, 12); Alfonso Muñoz Lopez (Hospital Carlos Haya, 10); Luis Ramos-Gómez (Hospital General de La Palma, 6); Victoria de la Torre-Prados (Hospital Universitario Virgen de la Victoria, 5); Romero Pellejero (Hospital General Yagüe, 4).
Sri Lanka (132 patients): Véronique Laloë (national coordinator), Bernhard Mandrella, Suganthan (Batticaloa General Hospital-Médecins Sans Frontières, 84); Sunil Perera (National Hospital of Sri Lanka, 39); Véronique Laloë, Kanapathipillai Mahendran (Point-Pedro Base Hospital, 9).
Switzerland (160 patients): Reto Stocker (national coordinator), Silke Ludwig (national coordinator) (University Hospital of Zurich, 133); Heinz Zimmermann (University Hospital Bern, 15); Urs Denzler (Kantonsspital Schaffhausen, 12).
Thailand (579 patients): Surakrant Yutthakasemsunt (national coordinator), Warawut Kittiwattanagul, Parnumas Piyavechvirat, Pojana Tapsai, Ajchara Namuang-jan (Khon Kaen Regional Hospital, 535); Upapat Chantapimpa (Chiangrai Prachanuko Hospital, 12); Chanothai Watanachai, Pusit Subsompon (Rayong Hospital, 11); Wipurat Pussanakawatin, Pensri Khunjan (Krabi Hospital, 10); Sakchai Tangchitvittaya, Somsak Nilapong (Suratthani Hospital, 8); Tanagorn Klangsang, Wibul Taechakosol (Roi Et Hospital, 2); Atirat Srinat (Lampang Hospital, 1).
Tunisia (63 patients): Zouheir Jerbi (national coordinator), Nebiha Borsali-Falfoul, Monia Rezgui (Hospital Habib Thameur, 63).
Turkey (2 patients): Nahit Cakar (Istanbul Medical Faculty, 2).
Uganda (43 patients): Hussein Ssenyonjo, Olive Kobusingye (Makerere Medical School, 43).
UK (1391 patients): Gabrielle Lomas, David Yates, Fiona Lecky (Hope Hospital, 209); Anthony Bleetman, Alan Baldwin, Emma Jenkinson, Shiela Pantrini (Birmingham Heartlands Hospital, 123); James Stewart, Nasreen Contractor, Trudy Roberts, Jim Butler (North Manchester General Hospital, 85); Alan Pinto, Diane Lee (Royal Albert Edward Infirmary, 83); Nigel Brayley, Karly Robbshaw, Clare Dix (Colchester General Hospital, 79); Sarah Graham, Sue Pye (Whiston Hospital, 69); Marcus Green, Annie Kellins (Selly Oak Hospital, 61); Chris Moulton, Barbara Fogg (Royal Bolton Hospital, 51); Rowland Cottingham, Sam Funnell, Utham Shanker (Eastbourne District General Hospital, 50); Claire Summers, Louise Malek (Trafford General Hospital, 41); Rowland Cottingham (national coordinator), Christopher Ashcroft, Jacky Powell (Royal Sussex County Hospital, 38); Steve Moore, Stephanie Buckley (Countess of Chester Hospital, 36); Mandy Grocutt, Steve Chambers (Worthing Hospital, 34); Amanda Morrice, Helen Marshall (Medway Maritime Hospital, 29); Julia Harris, Wendy Matthews, Jane Tippet (Chelsea and Westminster Hospital, 28); Simon Mardell, Fiona MacMillan, Anita Shaw (Furness General Hospital, 27); Pramod Luthra, Gill Dixon (Royal Oldham Hospital, 26); Mohammed Ahmed, John Butler, Mike Young (Stepping Hill Hospital, 26); Sue Mason, Ian Loveday (Northern General Hospital, 25); Christine Clark, Sam Taylor (Blackburn Royal Infirmary, 23); Paul Wilson (Cheltenham General Hospital, 23); Kassim Ali, Stuart Greenwood (Fairfield General Hospital, 23); Martin White, Rosa Perez (Queen Elizabeth the Queen Mother Hospital, 21); Sam Eljamel (Ninewells Hospital and Medical School, 19); Jonathan Wasserberg, Helen Shale (Queen Elizabeth Hospital Birmingham, 18); Colin Read, John McCarron (Russell’s Hall Hospital, 18); Aaron Pennell (Princess Alexandra Hospital, 16); Gautam Ray (Princess Royal Hospital, 14); John Thurston, Emma Brown (Darent Valley Hospital, 13); Lawrence Jaffey, Michael Graves (Royal Liverpool University Hospital, 12); Richard Bailey, Nancy Loveridge (Chesterfield and North Derbyshire Royal Hospital, 10); Geraint Evans, Shirleen Hughes, Major Kafeel Ahmed (Withybush General Hospital, 10); Jeremy Richardson, Claire Gallagher (Aberdeen Royal Infirmary, 8); Titus Odedun, Karen Lees (Ormskirk and District General Hospital, 8); David Foley, Nick Payne (Queen Mary’s Hospital, 8); Alan Pennycook, Carl Griffiths (Arrowe Park Hospital, 6); David Moore, Denise Byrne (City Hospital Birmingham, 5); Sunil Dasan (St Helier Hospital, 4); Ashis Banerjee, Steve McGuinness (Whittington Hospital, 4); Claude Chikhani (Doncaster Royal Infirmary, 2); Nigel Zoltie, Ian Barlow (Leeds General Infirmary, 2); Ian Stell (Bromley Hospital, 1); William Hulse, Jacqueline Crossley (Harrogate District Hospital, 1); Laurence Watkins (Institute of Neurology, 1); Balu Dorani (Queen Elizabeth Hospital Gateshead, 1).
Vietnam (2 patients)—Truong Van Viet (Cho Ray Hospital, 2).
Contributors: The writing committee comprised Pablo Perel (Chair), Miguel Arango, Tim Clayton, Phil Edwards, Edward Komolafe, Stuart Poccock, Ian Roberts, Haleema Shakur, Ewout Steyerberg, and Surakrant Yutthakasemsunt
Funding: The MRC CRASH trial was funded by the UK Medical Research Council .
Competing interests: None declared.
Ethical approval: All MRC CRASH collaborators obtained local ethics or research committee approval.
Provenance and peer review: Not commissioned; externally peer reviewed.
- 1. Bruns J Jr, Hauser WA. The epidemiology of traumatic brain injury: a review. Epilepsia 2003;44(suppl 10):2-10. [ DOI ] [ PubMed ] [ Google Scholar ]
- 2. Fleminger S, Ponsford J. Long term outcome after traumatic brain injury. BMJ 2005;331:1419-20. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 3. Hofman K, Primack A, Keusch G, Hrynkow S. Addressing the growing burden of trauma and injury in low- and middle-income countries. Am J Public Health 2005;95:13-7. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 4. Perel P, Wasserberg J, Ravi RR, Shakur H, Edwards P, Roberts I. Prognosis following head injury: a survey of doctors from developing and developed countries. J Eval Clin Pract 2007;13:464-5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. Lee KL, Pryor DB, Harrell FE Jr, Califf RM, Behar VS, Floyd WL, et al. Predicting outcome in coronary disease. Statistical models versus expert clinicians. Am J Med 1986;80:553-60. [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Murray LS, Teasdale GM, Murray GD, Jennett B, Miller JD, Pickard JD, et al. Does prediction of outcome alter patient management? Lancet 1993;341:1487-91. [ DOI ] [ PubMed ] [ Google Scholar ]
- 7. Perel P, Edwards P, Wentz R, Roberts I. Systematic review of prognostic models in traumatic brain injury. BMC Med Inform Decis Mak 2006;6:38. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 8. CRASH Trial Collaborators. Effect of intravenous corticosteroids on death within 14 days in 10 008 adults with clinically significant head injury (MRC CRASH trial): randomised placebo-controlled trial. Lancet 2004;364:1321-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. CRASH Trial Collaborators. Final results of MRC CRASH, a randomised placebo-controlled trial of intravenous corticosteroid in adults with head injury-outcomes at 6 months. Lancet 2005;365:1957-9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 10. Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet 1975;i:480-4. [ DOI ] [ PubMed ]
- 11. World Bank. World development indicators Washington, DC: World Bank, 2006
- 12. Brain Trauma Foundation (US) AAoNS. Management and prognosis of severe traumatic brain injury New York: 2000. www.braintrauma.org/site/DocServer/Prognosis_Guidelines_for_web.pdf?docID=241
- 13. Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996;15:361-87. [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Justice AC, Covinsky KE, Berlin JA. Assessing the generalizability of prognostic information. Ann Intern Med 1999;130:515-24. [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Maas AI, Marmarou A, Murray GD, Teasdale SG, Steyerberg EW. Prognosis and clinical trial design in traumatic brain injury: the IMPACT study. J Neurotrauma 2007;24:232-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 16. Hukkelhoven CW, Steyerberg EW, Rampen AJ, Farace E, Habbema JD, Marshall AF, et al. Patient age and outcome following severe traumatic brain injury: an analysis of 5600 patients. J Neurosurg 2003;99:666-73. [ DOI ] [ PubMed ] [ Google Scholar ]
- 17. Signorini DF, Andrews PJD, Jones PA, Wardlaw JM, Miller JD. Predicting survival using simple clinical variables: a case study in traumatic brain injury. J Neurol Neurosurg Psychiatry 1999;66:20-5. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 18. Maas AI, Hukkelhoven CW, Marshall LF, Steyerberg EW. Prediction of outcome in traumatic brain injury with computed tomographic characteristics: a comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery 2005;57:1173-82. [ DOI ] [ PubMed ] [ Google Scholar ]
- 19. Wardlaw JM, Easton VJ, Statham P. Which CT features help predict outcome after head injury? J Neurol Neurosurg Psychiatry 2002;72:188-92. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 20. Hukkelhoven CW, Steyerberg EW, Farace E, Habbema JD, Marshall LF, Maas AI. Regional differences in patient characteristics, case management, and outcomes in traumatic brain injury: experience from the tirilazad trials. J Neurosurg 2002;97:549-57. [ DOI ] [ PubMed ] [ Google Scholar ]
- 21. Hukkelhoven CW, Steyerberg EW, Habbema JD, Farace E, Marmarou A, Murray GD, et al. Predicting outcome after traumatic brain injury: development and validation of a prognostic score based on admission characteristics. J Neurotrauma 2005;22:1025-39. [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. Altman DG. Systematic reviews in health care: Systematic reviews of evaluations of prognostic variables. BMJ 2001;323:224-8. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
- View on publisher site
- PDF (212.7 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections

IMAGES
VIDEO
COMMENTS
The Medical Research Council (MRC) CRASH (corticosteroid randomisation after significant head injury) trial is the largest clinical trial conducted in patients with traumatic brain injury and presents a unique opportunity to develop a prognostic model.8 9 The trial prospectively included patients within eight hours of the injury, used ...
These prognostic models may be used as an aid to estimate mortality at 14 days and death and severe disability at six months in patients with traumatic brain injury (TBI). The predictions are based on the average outcome in adult patients with Glasgow coma score (GCS) of 14 or less, within 8 hours of injury, and can only support - not replace ...
Conflicts of interest: The CRASH Trial is sponsored by the Medical research Council (MRC) and not the manufactures of methyprednisolone. MRC funding covers meetings and central organisational costs only. Pharmacia Corporation are donoting drug and placebo, but the design, management, and finance of the study are entirely independent of them.
The trial is recruiting from 200 hospitals in 50 countries with another 100 centres planning to join the trial. The target for recruitment is 20,000 patients by 2006. The trial is wholly funded by the Medical Research Council of Great Britain and is multidisciplinary, involving doctors and nurses from a range of specialities.
A collaboration that commenced with the Medical Research Council of Great Britain (MRC), CRASH, led to the presentation of an enhanced prognostic calculator that included predictors of outcome in three prognostic models of increasing complexity; a Core model based on injury severity and demographics (including age, motor score and pupillary ...
The second was produced in 2008 from the 2004 Medical Research Council CRASH ... Use of the CRASH study prognosis calculator in patients with severe traumatic brain injury treated with an intracranial pressure-targeted therapy. J. Clin. Neurosci., 20 (2013), ...
Trial) database. The MRC CRASH data enabled the collaborators to produce a web-based calculator that formulates a percentage prediction of 14-day mortality and risk of unfavourable outcome at six months post-injury. The calculator adapts depending on whether CT data is available and is therefore of use in both high- and low-middle income countries.
The CRASH calculator was developed based on the Medical Research Council CRASH trial, which investigated the effect of corticosteroids on death and disability of patients with TBI. 7 Prognostic variables included age, sex, cause of trauma, time between trauma and randomization, Glasgow Coma Scale (GCS) score, pupil reactivity, computed ...
The country chosen as input in the prognosis calculator was Sweden. The CRASH prognosis calculator predicts for the individual patient the risk as a percentage with the 95% confidence interval (CI) of mortality at 14 days after the injury, and the risk of an unfavourable outcome, i.e. GOS 1-3, as a percentage with the 95% CI at 6 months after ...
The Medical Research Council (MRC) CRASH (corticosteroid randomisation after significant head injury) trial is the largest clinical trial conducted in patients with traumatic brain injury and presents a unique opportunity to develop a prognostic model. 8 9 The trial prospectively included patients within eight hours of the injury, used ...