Affiliations
- 1 Mayo Clinic
- 2 Sparrow Hospital
- 3 McLaren Macomb Regional Medical Center
- PMID: 30725971
- Bookshelf ID: NBK537286
Chlamydia is a sexually transmitted infectious disease caused by the bacterium Chlamydia trachomatis . In the United States, it is the most commonly reported bacterial infection. Globally, it is the most common sexually transmitted infection. It causes an ocular infection called "trachoma," which is the leading infectious cause of blindness worldwide.
In females, the cervix is the anatomic site that is most commonly infected. This can manifest as cervicitis, urethritis, pelvic inflammatory disease, perihepatitis, or proctitis. Chlamydial infections in women, especially if untreated, increase the risk of infertility and ectopic pregnancy, leading to high medical costs. There are also risks if a woman has an infection during pregnancy. Additionally, infants born vaginally to mothers infected with genital Chlamydia trachomatis may develop conjunctivitis and/or pneumonia.
In men, infection with Chlamydia trachomatis can lead to urethritis, epididymitis, prostatitis, proctitis, or reactive arthritis. Both men and women infected with C. trachomatis may also experience conjunctivitis, pharyngitis, and lymphogranuloma venereum. Lymphogranuloma venereum (LGV), caused by distinct serovars of Chlamydia trachomatis , is a less common disease characterized by enlarged lymph nodes or severe proctocolitis.
Copyright © 2024, StatPearls Publishing LLC.
- Continuing Education Activity
- Introduction
- Epidemiology
- Pathophysiology
- Histopathology
- History and Physical
- Treatment / Management
- Differential Diagnosis
- Treatment Planning
- Toxicity and Adverse Effect Management
- Complications
- Deterrence and Patient Education
- Enhancing Healthcare Team Outcomes
- Review Questions

Publication types
- Study Guide
Chlamydia Trachomatis Infection: Epidemiology, Prevention, Clinical, and Basic Science Research
Loading... Editorial 07 March 2023 Editorial: Chlamydia trachomatis infection: Epidemiology, prevention, clinical, and basic science research Changchang Li , Jason Ong , Weiming Tang and Cheng Wang 1,962 views 0 citations
Original Research 13 January 2023 Determinants and prediction of Chlamydia trachomatis re-testing and re-infection within 1 year among heterosexuals with chlamydia attending a sexual health clinic Xianglong Xu , 8 more and Jason J. Ong 2,507 views 4 citations
Original Research 09 December 2022 The association between adverse pregnancy outcomes with genital Chlamydia Trachomatis infection among pre-pregnancy couples in Shenzhen, China: A cross-sectional study Si Sun , 7 more and Zhenzhou Luo 1,680 views 0 citations
Original Research 23 November 2022 High chlamydia infection and its associated factors among patients seeking clinic-based STI services in Southern China: A preliminary cross-sectional study Honglin Wang , 9 more and Yumao Cai 1,199 views 5 citations
Original Research 17 November 2022 Cluster analysis for symptomatic management of Neisseria gonorrhoea and Chlamydia trachomatis in sexually transmitted infections related clinics in China Ning Ning , 7 more and Yumao Cai 1,511 views 1 citations
Original Research 17 November 2022 Acceptability of rectal self-sampling in non-clinical venues for chlamydia and gonorrhea testing among men who have sex with men: A cross-sectional study in Shenzhen, China Rongxing Weng , 7 more and Yumao Cai 1,298 views 5 citations
Loading... Original Research 31 October 2022 Prevalence of syphilis and chlamydia trachomatis infection among female sex workers in Jiangsu, China: Results from a multicenter cross-sectional and venue-based study Lingen Shi , 11 more and Gengfeng Fu 2,591 views 5 citations
Original Research 31 October 2022 Distribution of Chlamydia trachomatis ompA genotypes and its association with abnormal cervical cytology among women of reproductive age in Shenzhen, China Lan-lan Liu , 6 more and Zhen-zhou Luo 1,565 views 3 citations
Original Research 28 October 2022 Real-life cohort experience after implementing HIV pre-exposure prophylaxis for one year in northwest Spain Alexandre Pérez-González , 8 more and Eva Poveda 1,788 views 2 citations
Loading... Original Research 11 October 2022 Prevalence of syphilis and chlamydia trachomatis infection among men who have sex with men in Jiangsu province, China: A cross-sectional survey Haiyang Hu , 10 more and Gengfeng Fu 2,253 views 6 citations
Original Research 10 October 2022 Repeat infections with chlamydia in women may be more transcriptionally active with lower responses from some immune genes Wilhelmina M. Huston , 12 more and Jane S. Hocking 1,597 views 1 citations
Loading... Original Research 26 September 2022 Rhein inhibits Chlamydia trachomatis infection by regulating pathogen-host cell Xueying Yu , 8 more and Yaohua Xue 9,266 views 5 citations
Loading... Original Research 27 July 2022 Evaluating the impact and cost-effectiveness of chlamydia management strategies in Hong Kong: A modeling study Sandra Montes-Olivas , 8 more and Jason J. Ong 4,814 views 2 citations
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List
Web‐based chlamydia education for university students: A pilot project
Nancy g russell, phyllis w sharps, elizabeth sloand.
- Author information
- Article notes
- Copyright and License information
Correspondence , Nancy G. Russell, Johns Hopkins University School of Nursing, 525 N Wolfe Street, Baltimore, Maryland, 21205, Email: [email protected]
Corresponding author.
Revised 2022 Jan 13; Received 2021 Sep 22; Accepted 2022 May 10; Collection date 2022 Sep.
This is an open access article under the terms of the http://creativecommons.org/licenses/by-nc-nd/4.0/ License, which permits use and distribution in any medium, provided the original work is properly cited, the use is non‐commercial and no modifications or adaptations are made.
Chlamydia disproportionately affects individuals aged 15–24 years. A lack of chlamydia knowledge in this high‐risk group likely contributes to decreased testing, but interventions to increase chlamydia knowledge in this population are not well‐described in the literature. The purpose of this pilot project was to increase chlamydia knowledge in a sample of university students using nurse‐developed web‐based education.
A pre‐ and post‐test design was used to evaluate participant knowledge of chlamydia before and after completing a nurse‐developed web‐based education intervention designed for university students.
Forty‐seven undergraduate students at one U.S. university participated. A focus group and scientific evidence informed the development of the web‐based education.
Participants had a significant increase in chlamydia knowledge after completing the online educational intervention ( M = 8.0, SD = 0.000) compared to baseline ( M = 6.5, SD = 1.5), t (33) = −5.821, p < .0001. Pilot results provide promising evidence that web‐based nurse‐developed education designed specifically for university students can increase chlamydia knowledge.
Keywords: adolescent health, chlamydia, health education, nurses, sexual health promotion, university students, web‐based education, young adults
What problem did the study address?
Reducing the proportion of adolescents and young adults with chlamydial infections is a global health priority, and inadequate chlamydia knowledge in this population likely contributes to decreased chlamydia testing. This pilot project provides preliminary results that web‐based education designed specifically for university students can increase their knowledge of chlamydia. Future studies with larger and more diverse samples in a variety of health settings where adolescents and young adults seek care are needed to further evaluate the positive effects of this web‐based education approach.
1. INTRODUCTION AND BACKGROUND
Sexually transmitted infections (STIs) are infections spread predominantly by sexual contact (World Health Organization [WHO], 2021 ). Some STIs, including chlamydia, can also be transmitted via the maternal‐foetal route (WHO, 2021 ). The WHO estimates that, worldwide, over one million curable STIs are contracted daily, with chlamydia being among the most prevalent (2018). In the United States, chlamydia is the most commonly reported bacterial STI, with the highest rates being among adolescents and young adults (AYA) aged 15–24 years – a population that includes many university students (Centers for Disease Control and Prevention [CDC], 2021a ; Workowski et al., 2021 ). U.S. chlamydia rates continue to increase each year, with over 1.8 million cases reported in 2021 – an increase of 15% since 2015 (CDC, 2021b ). Chlamydia is also among the most costly STIs in the United States (Owusu‐Edsei et al., 2013 ).
Though some individuals with chlamydia may present with symptoms such as vaginal or urethral discharge, chlamydial infections are frequently asymptomatic, undiagnosed and untreated (CDC, 2021a , 2021b ). Untreated chlamydial infections can result in serious complications, including pelvic inflammatory disease, ectopic pregnancy, chronic pelvic pain and infertility, and an increased risk of HIV transmission or acquisition (CDC, 2021a , 2021b ; Workowski et al., 2021 ). Thus, early detection and treatment of chlamydia are crucial (CDC, 2021a ).
Although chlamydia screening has increased in recent years, many at high risk, such as young adults aged 18–24 years who are often university students, are still not being tested (CDC, 2021b). A lack of chlamydia knowledge (Friedman & Bloodgood, 2010 ), the associated stigma (Booth et al., 2012 ) and misconceptions regarding risk (Hickey & Cleland, 2013 ) likely contribute to decreased test‐seeking in this population. Even when AYA are aware of chlamydia and know it is a STI, they often lack sufficient knowledge of the disease and its significance (Keizur et al., 2021 ; Lorimer & Hart, 2010 ). University‐aged individuals may not seek chlamydia testing because of a decreased risk perception regarding the disease and its acquisition (Keizur et al., 2021 ), including related lack of knowledge and misbelief about STI risk based on their type of sexual behaviour (i.e., only oral intercourse) (Downing‐Matibag & Geisinger, 2009 ). Trust in a sexual partner also contributes to decreased STI risk perception (Masaro et al., 2008 ). Further, even after AYA are diagnosed with a STI, a discrepancy in perceived versus actual risk may persist (Hickey & Cleland, 2013 ).
Interventions to increase chlamydia knowledge targeted specifically for university‐aged individuals may encourage increased testing and are needed (Denison et al., 2018 ; Keizur et al., 2021 ; Sagor et al., 2016 ). AYA, including university students, report insufficient sexual health education and a desire for more and better sexual health education (Denison et al., 2018 ; Lederer & Sheena, 2020 ; Normansell et al., 2016 ). International leaders in AYA health strongly endorse comprehensive sexuality education, with STIs being a key issue (United Nations Educational, Scientific and Cultural Organization [UNESCO], 2018 ). Sexuality education has many positive effects, including increasing knowledge, but is lacking worldwide (UNESCO, 2018 ), and high chlamydia rates persist despite current efforts. Considering and incorporating the learning needs and preferences of AYA in the development of educational interventions targeting this population is key to effectively meet their needs and increase their knowledge (Holstrom, 2015 ; von Rosen et al., 2017 ). Interventions that normalize chlamydia screening, as well as increase awareness of the commonality of chlamydia, are important in this population (Booth et al., 2012 ; Denison et al., 2018 ). The use of simple and understandable language, a clear layout and a credible information source, such as a nurse, are also important for educational interventions targeting AYA (von Rosen et al., 2017 ).
The Internet is a common source of information for AYA, but accessing accurate and valid sexual health information may be challenging for this group, and inaccurate information may contribute to unhealthy sexual behaviour (von Rosen et al., 2017 ). Nurses and other trustworthy healthcare professionals should work to find effective ways to disseminate accurate and reliable sexual health information to AYA (von Rosen et al., 2017 ). Many AYA have access to smartphones or computers, so interventions that use web‐based technologies are promising (Lederer & Sheena, 2020 ; Sagor et al., 2016 ; Shafii et al., 2014 ). Use of these technologies can address documented AYA barriers to STI knowledge and testing, including confidentiality and ease of access (Cuffe et al., 2016 ; Friedman & Bloodgood, 2010 ; Normansell et al., 2016 ). The persistent lack of sexual health and chlamydia knowledge among university‐aged individuals suggests a need for new and creative strategies to provide sexual health education for this population (Denison et al., 2018 ; Lederer & Sheena, 2020 ).
To be effective, sexual health education for AYA should be designed to meet their learning needs and preferences (Holstrom, 2015 ; von Rosen et al., 2017 ), but there is a dearth of current web‐based interventions in the existing literature that focus specifically on chlamydia and address the learning needs and preferences of AYA. Given the high rates of chlamydia worldwide and significant burden of disease among the university‐aged population, new, creative and easily accessible educational interventions designed to meet the learning needs of this group on this topic are paramount (Keizur et al., 2021 ). The purpose of this pilot project was to extend the literature and increase chlamydia knowledge in a sample of university students using nurse‐developed web‐based education designed to incorporate and meet the learning needs of this population.
1.1. Design
We used a pre/post‐test design and aimed to increase chlamydia knowledge among undergraduate students through web‐based education in the fall 2018 semester. The setting was a mid‐size, private, co‐educational, U.S. university. A convenience sample of students was recruited via flyers and emails. Undergraduate students aged 18–24 years were eligible. Participation was voluntary.
1.2. Ethics
The university’s Institutional Review Board approved this project as exempt research.
A focus group of upperclassmen was used to assess their chlamydia knowledge as freshmen and to gather information regarding their learning preferences. The goal of the focus group was to inform the development of the web‐based education, so questions were limited to those regarding chlamydia knowledge and test‐seeking behaviour, and learning needs and preferences, rather than actual sexual or testing experiences (See Appendix S1 ). The target population for the educational intervention was initially freshmen, as evidence suggests younger college‐aged individuals are more likely to engage in risky sexual behaviour and have increased risk for chlamydia and PID (Downing‐Matibag & Geisinger, 2009 ; Habel et al., 2016 ). An in‐person focus group with two upperclassmen assembled on campus and used a semi‐structured interview format with open‐ended questions. Participants' self‐selected pseudonyms and responses were recorded via typed notes. Students unable to participate in the focus group were offered an opportunity to independently complete an anonymous online survey using the same in‐person focus group questions. The combined sample of in‐person and online focus group participants consisted of two females and one male.
Focus group findings were discussed until all investigators reached consensus. The major themes that emerged from the focus group were the need for all students to receive chlamydia education (not just freshmen), a belief that participation would be higher with web‐based education, and a lack of chlamydia knowledge among students. These findings were integrated into the educational intervention. Additionally, the educational intervention integrated CDC guidelines (2016), National Chlamydia Coalition information ( 2018 ) and studies of STI knowledge or testing in AYA (Sagor et al., 2016 ). The intervention included evidence‐based information about chlamydia prevalence, risk, transmission, symptoms, complications, testing and treatment. Clinical experts established the content validity of the intervention and pre/post assessments.
The 20 minute web‐based intervention consisted of: (a) evidence‐based pre/post‐intervention knowledge assessments (each 10 questions) that evaluated learning objectives and explored perceptions of testing barriers and facilitators (Booth, 2012 ; Sagor et al., 2016 ), and (b) replayable video education that included chlamydia disease information, testing resources and case scenarios. Pre‐ and post‐session assessments evaluated the learning objectives and were adapted from those used by Sagor et al. ( 2016 ) and presented in a “Yes, No, Not Sure” format, where the correct answer was scored as 1, and the incorrect answer (including “Not Sure”) was scored as 0. Two questions explored student perceptions of barriers and facilitators to chlamydia testing (Booth et al., 2012 ). The intervention and assessments were delivered online and easily accessed by participants through an anonymous hyperlink or QR code on a smart device or computer. Focus group participants received a snack and intervention participants could enter a $25 gift card raffle upon intervention completion.
2.1. Data analysis
SPSS, version 25.0, was used for quantitative data analysis. Analysis included descriptive and inferential statistics. A p ‐value of <.05 was considered statistically significant. A formal power analysis was not performed for this project as it was a pilot project. Overall change from in chlamydia knowledge before and after the intervention was evaluated with a paired t ‐test for continuous scores. A McNemar’s test was planned to evaluate knowledge changes for individual items that were dichotomous.
The sample size included 44 students ( N = 44). Ten (22.7%) completed only the pre‐intervention assessment and were excluded from knowledge change analyses; 34 (77.2%) completed the pre‐ and post‐intervention assessments. The mean age of participants was 20 years, and the majority identified as female.
Participants who completed the pre‐ and post‐intervention assessments ( n = 34) had a significant post‐intervention increase in chlamydia knowledge ( M = 8.0) compared to baseline ( M = 6.5), t (33) = −5.821, p < .0001). At baseline, participants had the least knowledge regarding chlamydia disease commonality, testing and treatment processes, presentation in men and potential complications (Table 1 ). The planned McNemar’s test was not conducted as all participants ( n = 34) scored 100% on all items on the post‐intervention assessment.
Pre‐ and post‐intervention chlamydia knowledge assessment outcomes
Abbreviation: SD , standard deviation.
p < .05.
Most participants reported they would seek chlamydia testing if they were sexually active pre‐ ( n = 34, 88.2%) and post‐intervention ( n = 34, 97.1%). Participant‐identified facilitators for chlamydia testing included inexpensive or free testing, easy access and increased knowledge regarding STIs and testing recommendations.
4. DISCUSSION
Similar to other studies that evaluated the feasibility, acceptance and/or effectiveness of computer‐based education to increase chlamydia and/or sexual health knowledge among AYA (Sagor et al., 2016 ; Shafii et al., 2014 ), this pilot project provides evidence that web‐based education for university students is a feasible and acceptable strategy for increasing chlamydia knowledge in this high‐risk population. Further, our evidenced‐based approach to involve students and incorporate their learning needs and preferences in our intervention, as recommended by the literature (Holstrom, 2015 ; von Rosen et al., 2017 ), resulted in nurse‐developed web‐based education that was feasible and acceptable to this population, and increased chlamydia knowledge among the participants.
At baseline, participants in our project knew that chlamydia testing was important but lacked sufficient knowledge about the testing and treatment processes and the disease significance, which is similar to other research demonstrating that even if AYA are aware of chlamydia as a STI, they often lack sufficient knowledge of the disease (Keizur et al., 2021 ; Lorimer & Hart, 2010 ). Nurses caring for university students and other AYA should be aware of these chlamydia knowledge gaps and not assume that knowledge of the importance of chlamydia testing or awareness of chlamydia as a STI equates to adequate knowledge needed to reduce risk. Further, lack of knowledge was a commonly reported barrier to testing among our participants, even after completing the web‐based education intervention, suggesting, in line with other research (Lederer & Sheena, 2020 ), that continued chlamydia education for college‐aged individuals is needed.
Innovative, evidence‐based online interventions that are easily accessible, confidential and aimed at increasing sexual health knowledge in AYA, such as this pilot, are important (Holstrom, 2015 ; Lederer & Sheena, 2020 ; von Rosen et al., 2017 ). Nurses must recognize the popularity of web technology among AYA and consider using these platforms for health education. Web‐based education can also be effective for reaching AYA when face‐to‐face interventions are not possible, including during the current COVID‐19 pandemic. This pilot intervention was inexpensive, easy to access, and could be effective in other settings where AYA seek care. Replication of this pilot with larger and more diverse samples would further evaluate and validate the effectiveness of this approach. Continued development of innovative technology‐based education solutions for AYA, including evaluating the relationship between increased chlamydia knowledge and testing uptake, is warranted.
4.1. Limitations
Though this was a pilot project, the small sample size and lack of evaluating long‐term chlamydia knowledge retention are limitations. Recruitment constraints imposed by the institution limited the ability to target all undergraduate students for participation and resulted in using a convenience sample. The institution aimed to protect participants from disclosing sensitive sexual health information, and assessing actual sexual behaviours was not possible. The reported hypothetical participant behaviour may not have reflected actual participant behaviour. Additionally, this pilot project did not evaluate the effects of increased knowledge or other factors (e.g., cost) on actual chlamydia testing uptake. Lastly, though the web‐based education and pre‐ and post‐assessments for this pilot project were derived from evidence and scientific studies, more work is needed to determine their reliability and validity for a larger and more diverse sample including a comparison group.
5. CONCLUSION
Reducing the proportion of AYA with chlamydia is a global health priority (WHO, 2018 ). Inadequate chlamydia knowledge among AYA likely contributes to decreased testing (Friedman & Bloodgood, 2010 ). Interventions that aim to increase chlamydia knowledge and incorporate learning needs and preferences of AYA that are feasible and acceptable are needed (Denison et al., 2018 ; Holstrom, 2015 ; Keizur et al., 2021 ; von Rosen et al., 2017 ). This pilot project provides preliminary results that nurse‐developed web‐based education designed specifically for university students can increase their chlamydia knowledge and is feasible and acceptable.
AUTHOR CONTRIBUTIONS
NR, PS: Made substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data. NR, PS, BS: Involved in drafting the manuscript or revising it critically for important intellectual content. Given final approval of the version to be published. NR, PS, BS: Each author should have participated sufficiently in the work to take public responsibility for appropriate portions of the content. NR, PS, BS: Agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
CONFLICT OF INTEREST
No conflict of interest has been declared by the authors.
PATIENT CONSENT STATEMENT
No patient consent was needed for this project.
Supporting information
Appendix S1
ACKNOWLEDGEMENTS
The authors would like to thank and acknowledge the contributions and support of Dr. Vincent J. WinklerPrins, MD and Dr. Elisa DeAngelis, MD to this work.
Russell, N. G. , Sharps, P. W. , & Sloand, E. (2022). Web‐based chlamydia education for university students: A pilot project. Nursing Open, 9, 2342–2347. 10.1002/nop2.1244
Funding Information
Georgetown University in Washington, D.C. provided approximately $125 for this project, which was used to purchase Amazon gift cards that were raffled off to participants. No other specific grant from any funding agency in the public, commercial or not‐for‐profit sectors was received
DATA AVAILABILITY STATEMENT
Supportive de‐identified data for this project are available from the authors upon a reasonable request for 5 years after publication.
- Booth, A. R. , Harris, P. R. , Goyder, E. , & Norman, P. (2012). Beliefs about chlamydia testing amongst young people living in relatively deprived areas. Journal of Public Health (Oxf), 35(2), 213–222. 10.1093/pubmed/fds082 [ DOI ] [ PubMed ] [ Google Scholar ]
- Centers for Disease Control and Prevention . (2021a). Chlamydia CDC fact sheet. Available from: https://www.cdc.gov/std/chlamydia/stdfact‐chlamydia‐detailed.htm .
- Centers for Disease Control and Prevention . (2021b). Sexually transmitted disease surveillance 2019. U.S. Department of Health and Human Services. https://www.cdc.gov/std/statistics/2019/default.htm [ Google Scholar ]
- Cuffe, K. M. , Newton‐Levinson, A. , Gift, T. L. , McFarlane, M. , & Leichliter, J. S. (2016). Sexually transmitted infection testing among adolescents and young adults in the United States. Journal of Adolescent Health, 58, 512–519. 10.1016/j.jadohealth.2016.01.002 [ DOI ] [ PubMed ] [ Google Scholar ]
- Denison, H. J. , Bromhead, C. , Grainger, R. , Dennison, E. M. , & Jutel, A. (2018). What influences university students to seek sexually transmitted infection testing?: A qualitative study in New Zealand. Sexual & Reproductive Healthcare, 16, 56–60. 10.1016/j.srhc.2018.01.004 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Downing‐Matibag, T. M. , & Geisinger, B. (2009). Hooking up and sexual risk taking among college students: A health belief model perspective. Qualitative Health Research, 19(9), 1196–1209. 10.1177/1049732309344206 [ DOI ] [ PubMed ] [ Google Scholar ]
- Friedman, A. L. , & Bloodgood, B. (2010). Something we’d rather not talk about: Findings from CDC exploratory research on sexually transmitted disease communication with girls and women. Journal of Women’s Health (Larchmt), 19(10), 1823–1831. 10.1089/jwh.2010.1961 [ DOI ] [ PubMed ] [ Google Scholar ]
- Habel, M.A. , Leichliter, J.S. & Torrone, E. (2016). Exploring chlamydia positivity among females on college campuses, 2008‐2010 [published online January 5, 2016]. Journal of American College Health, 64(6), 496–501. 10.1080/07448481.2015.1117470. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hickey, M.T. & Cleland, C. (2013). Sexually transmitted infection risk perception among female college students [published online October 11, 2012]. Journal of the American Association of Nurse Practitioners, 25(7), 377–384. 10.1111/j.17457599.2012.00791.x [ DOI ] [ PubMed ] [ Google Scholar ]
- Holstrom, A. H. (2015). Sexuality education goes viral: What we know about online sexual health information. American Journal of Sexuality Education, 10(3), 277–294. 10.1080/15546128.2015.1040569 [ DOI ] [ Google Scholar ]
- Keizur, E. M. , Bristow, C. C. , Baik, Y. , & Kalusner, J. D. (2021). Knowledge and testing preferences for chlamydia trachomatis, Neisseria gonorrhoeae, and trichomonas vaginalis infections among female undergraduate students. Journal of American College Health, 68(7), 754–761. 10.1080/07448481.2019.1616742 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lederer, A. M. , & Sheena, B. S. (2020). Analysis of college students' gaps in knowledge about sexually transmitted infections. Health Education Journal, 80(2), 238–250. 10.1177/0017896920959091 [ DOI ] [ Google Scholar ]
- Lorimer, K. , & Hart, G. J. (2010). Knowledge of chlamydia trachomatis among men and women approached to participate in community‐based screening, Scotland, UK. BMC Public Health, 10(794). 10.1186/1471-2458-10-794 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Masaro, C. L. , Dahinten, V. S. , Johnson, J. , Ogilvie, G. , & Patrick, D. M. (2008). Perceptions of sexual partner safety. Sexually Transmitted Diseases, 35(6), 566–571. 10.1097/OLQ.0b013e3181660c43 [ DOI ] [ PubMed ] [ Google Scholar ]
- National Chlamydia Coalition . (2018). Chlamydia 101. Available from: http://chlamydiacoalition.org/chlamydia‐101/ .
- Normansell, R. , Drennan, V. M. , & Oakeshott, P. (2016). Exploring access and attitudes to regular sexually transmitted infection screening: The views of young, multi‐ethnic, inner‐city, female students. Health Expectations, 19(2), 322–330. 10.1111/hex.12354 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Owusu‐Edsei, K. , Chesson, H. W. , Gift, T. L. , Tao, G. , Mahajan, R. , Ocfemia, M. C. , & Kent, C. K. (2013). The estimated direct medical cost of selected sexually transmitted infections in the United States, 2008. Sexually Transmitted Infections, 40(3), 197–201. 10.1097/OLQ.0b013e318285c6d2 [ DOI ] [ PubMed ] [ Google Scholar ]
- Sagor, R. S. , Golding, J. , Giorgio, M. M. , & Blake, D. R. (2016). Power of knowledge: Effect of two educational interventions on readiness for chlamydia screening [published online September 8, 2015]. Clinical Pediatrics (Phila), 55(8), 717–723. 10.1177/0009922815604597 [ DOI ] [ PubMed ] [ Google Scholar ]
- Shafii, T. , Benson, S. K. , Morrison, D. M. , Hughes, J. P. , Golden, M. R. , & Holmes, K. K. (2014). Results from eKISS (electronic KIOSK intervention for safer‐sex): A pilot randomized controlled trial to test an interactive computer‐based intervention for sexual health in adolescents and young adults. Journal of Adolescent Health, 54(2), S10. 10.1016/j.jadohealth.2013.10.036 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- United Nations Educational, Scientific and cultural organization . (2018). International technical guidance on sexuality education : An evidence‐informed approach. Available from: https://unesdoc.unesco.org/in/documentViewer.xhtml?v=2.1.196&id=p::usmarcdef_0000260770&file=/in/rest/annotationSVC/DownloadWatermarkedAttachment/attach_import_d8d4de18‐19d0‐4a35‐8eb2‐ab5eaa5ca5d3%3F_%3D260770eng.pdf&locale=en&multi=true&ark=/ark:/48223/pf0000260770/PDF/260770eng.pdf#%5B%7B%22num%22%3A74%2C%22gen%22%3A0%7D%2C%7B%22name%22%3A%22XYZ%22%7D%2C0%2C842%2C0%5D
- von Rosen, A. J. , von Rosen, F. T. , Tinnemann, P. , & Müller‐Riemenschneider, F. (2017). Sexual health and the internet: Cross‐sectional study of online preferences among adolescents. Journal of Medical Internet Research, 19(11), e379. 10.2196/jmir.7068 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- World Health Organization . (2021). Sexually transmitted infections (STIs). Available from: https://www.who.int/news‐room/fact‐sheets/detail/sexually‐transmitted‐infections‐(stis)
- World Health Organization . (2018). Report on global sexually transmitted infection surveillance, 2018. Available from: https://apps.who.int/iris/bitstream/handle/10665/277258/9789241565691‐eng.pdf?sequence=5&isAllowed=y
- Workowski, K. A. , Bachmann, L. H. , Chan, P. A. , Johnston, C. M. , Muzny, C. A. , Park, I. , Reno, H. , Zenilman, J. M. , & Bolan, G. A. (2021). Sexually transmitted infections treatment guidelines, 2021. Morbidity and Mortality Weekly Report Recommendations and Reports, 70(4), 1–184. https://www.cdc.gov/std/treatment‐guidelines/STI‐Guidelines‐2021.pdf [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data availability statement.
- View on publisher site
- PDF (345.6 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Chlamydia cell biology and pathogenesis
Cherilyn elwell, kathleen mirrashidi, joanne engel.
- Author information
- Article notes
- Copyright and License information
Correspondence to J.E. [email protected]
Issue date 2016 Jun.
Chlamydia spp. are important causes of human disease for which no effective vaccine exists. These obligate intracellular pathogens replicate in a specialized membrane compartment and use a large arsenal of secreted effectors to survive in the hostile intracellular environment of the host. In this Review, we summarize the progress in decoding the interactions between Chlamydia spp. and their hosts that has been made possible by recent technological advances in chlamydial proteomics and genetics. The field is now poised to decipher the molecular mechanisms that underlie the intimate interactions between Chlamydia spp. and their hosts, which will open up many exciting avenues of research for these medically important pathogens.
Chlamydiae are Gram-negative, obligate intracellular pathogens and symbionts of diverse organisms, ranging from humans to amoebae 1 . The best-studied group in the Chlamydiae phylum is the Chlamydiaceae family, which comprises 11 species that are pathogenic to humans or animals 1 . Some species that are pathogenic to animals, such as the avian pathogen Chlamydia psittaci , can be transmitted to humans 1 , 2 . The mouse pathogen Chlamydia muridarum is a useful model of genital tract infections 3 . Chlamydia trachomatis and Chlamydia pneumoniae , the major species that infect humans, are responsible for a wide range of diseases 2 , 4 and will be the focus of this Review. Strains of C. trachomatis are divided into three biovars and are further subtyped by serovar. The trachoma biovar (serovars A–C) is the leading cause of non-congenital blindness in developing nations, whereas the genital tract biovar (serovars D–K) is the most prevalent sexually transmitted bacterium. In women, 70–80% of genital tract infections with C. trachomatis are asymptomatic, but 15–40% ascend to the upper genital tract, which can lead to serious sequelae, including pelvic inflammatory disease, infertility and ectopic pregnancy 4 . The lympho granuloma venereum (LGV) biovar (serovars L1–L3) causes invasive urogenital or anorectal infection, and in the past 10 years, the incidence of LGV in HIV-infected men who have sex with men has increased 5 . Infection with C. trachomatis also facilitates the transmission of HIV and is associated with cervical cancer 4 . C. pneumoniae causes respiratory infections, accounting for ~10% of community-acquired pneumonia, and is linked to a number of chronic diseases, including asthma, atherosclerosis and arthritis 1 , 2 . Although chlamydial infection is treatable with antibiotics, no drug is sufficiently cost-effective for the elimination of the bacterium in developing nations, and an effective vaccine has thus far been elusive 6 .
All chlamydiae share a developmental cycle in which they alternate between the extracellular, infectious elementary body and the intracellular, non-infectious reticulate body 7 ( FIG. 1 ). Elementary bodies enter mucosal cells and differentiate into reticulate bodies in a membrane bound compartment — the inclusion. After several rounds of replication, reticulate bodies re-differentiate into elementary bodies and are released from the host cell, ready to infect neighbouring cells.
Figure 1. The life cycle of C hlamydia trachomatis .
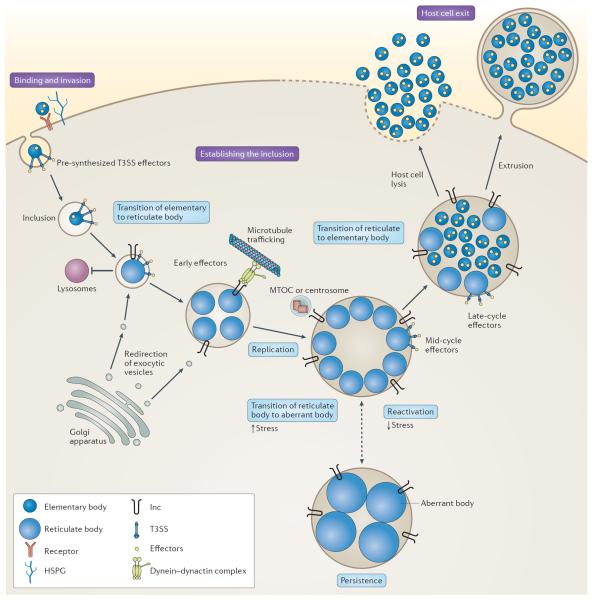
The binding of elementary bodies to host cells is initiated by the formation of a trimolecular bridge between bacterial adhesins, host receptors and host heparan sulfate proteoglycans (HSPGs). Next, pre-synthesized type III secretion system (T3SS) effectors are injected into the host cell, some of which initiate cytoskeletal rearrangements to facilitate internalization and/or initiate mitogenic signalling to establish an anti-apoptotic state. The elementary body is endocytosed into a membrane-bound compartment, known as the inclusion, which rapidly dissociates from the canonical endolysosomal pathway. Bacterial protein synthesis begins, elementary bodies convert to reticulate bodies and newly secreted inclusion membrane proteins (Incs) promote nutrient acquisition by redirecting exocytic vesicles that are in transit from the Golgi apparatus to the plasma membrane. The nascent inclusion is transported, probably by an Inc, along microtubules to the microtubule-organizing centre (MTOC) or centrosome. During mid-cycle, the reticulate bodies replicate exponentially and secrete additional effectors that modulate processes in the host cell. Under conditions of stress, the reticulate bodies enter a persistent state and transition to enlarged aberrant bodies. The bacteria can be reactivated upon the removal of the stress. During the late stages of infection, reticulate bodies secrete late-cycle effectors and synthesize elementary-body-specific effectors before differentiating back to elementary bodies. Elementary bodies exit the host through lysis or extrusion.
Chlamydiae have substantially reduced genomes (1.04 Mb encoding 895 open reading frames for C. trachomatis ) that lack many metabolic enzymes 8 , which makes these bacteria reliant on the host for many of their metabolic requirements. Approximately two-thirds of predicted proteins are shared across species, which reflects genetic conservation and the evolutionary constraints that are imposed by their intracellular lifestyle and conserved developmental cycle 1 , 9 . One exception is a region of high genomic diversity termed the `plasticity zone', which encodes an array of virulence factors, including cytotoxin, membrane attack complex/perforin protein (MACPF) and phospholipase D, which may have a role in host tropism and niche specificity 1 , 9 . Chlamydiae encode a large number of virulence effectors, which comprise ~10% of their genome 10 . These effectors are delivered through specialized secretion systems to the bacterial surface (by a type V secretion system (T5SS)), the inclusion lumen (by a type II secretion system (T2SS)), or into the host cell cytosol or inclusion membrane (by a type III secretion system (T3SS)) 11 ( BOX 1 ). In addition, most strains carry a plasmid that contributes to virulence 12 . In this Review, we summarize the current understanding of chlamydial biology, highlighting recent advances in host and bacterial cell biology, proteomics and chlamydial genetics, and areas that we expect to progress substantially in the coming decade.
Box 1 | Inclusion membrane proteins: a unique set of T3SS effectors.
The type III secretion system (T3SS) is a needle-like molecular syringe that enables the direct injection of bacterial effector molecules across host membranes 150 . Chlamydia spp. use the T3SS at various stages of infection, including during initial host cell contact with the plasma membrane and during the intracellular phase, in which effectors are injected into the cytosol of the host cell and can access other intracellular compartments, such as the nucleus 11 . The T3SS is spatially restricted in chlamydiae, with needle complexes localized to one pole of the elementary body 39 or concentrated at the site at which reticulate bodies contact the inclusion membrane 93 . Chlamydiae produce a unique family of T3SS effectors termed inclusion membrane proteins (Incs) 16 , 20 , of which there are 36–107 depending on the species 151 , 152 . These effectors are translocated across, and inserted into, the inclusion membrane, in which they are ideally positioned to mediate host–pathogen interactions 20 . The defining feature of Incs is one or more bilobed hydrophobic domains composed of two closely spaced membrane-spanning regions that are separated by a short hairpin loop, with their amino terminus and/or carboxyl terminus predicted to extend into the cytoplasm of the host cell 16 . Incs are primarily expressed early during infection, when they may be important in the establishment of the inclusion, and at mid-cycle, when they may be involved in the maintenance of the inclusion and the acquisition of nutrients 20 . Genome-wide comparisons reveal a core set of Incs that are shared across Chlamydia spp. as well as diverse species-specific Incs that may be key determinants of host tropism and site-specific disease 151 , 152 . Incs share little homology to each other or to other known proteins, with the exception of coiled-coil or soluble N -ethylmaleimide-sensitive factor attachment protein receptor (SNARE)-like domains, which provides limited insight into their functions 16 . Incs are hypothesized to recruit host proteins to the inclusion membrane to promote fusion with nutrient-rich compartments, inhibit fusion with degradative compartments, hijack host machinery or organelles, disrupt normal host pathways, or assemble novel complexes with new functions 20 . The Inc–host interactions identified thus far indicate that Incs participate in numerous processes, including the rearrangement of the host cell cytoskeleton, membrane dynamics, centrosome tethering, lipid acquisition and resistance to apoptosis 20 ( FIG 2a,b ). In addition, Incs form homotypic or heterotypic complexes on the surface of the inclusion 65 , 116 . Finally, Incs may provide structural stability to the growing inclusion membrane 153 . A large-scale proteomic screen of Incs in Chlamydia trachomatis has revealed putative host binding partners for approximately two-thirds of Incs 67 . Together with the recent description of the proteome of purified mid-cycle inclusions 68 , a comprehensive landscape of Inc–host interactions is developing.
The developmental cycle
Elementary bodies and reticulate bodies are morphologically and functionally distinct. Elementary bodies survive in the harsh extracellular environment; their spore-like cell wall is stabilized by a network of proteins that are crosslinked by disulfide bonds, termed the outer membrane complex, which confers resistance to osmotic stress and physical stress 13 . Although they were once considered to be metabolically inactive, studies using a host-free (axenic) system indicate that elementary bodies have high metabolic and biosynthetic activities and depend on d -glucose-6-phosphate as a source of energy 14 . Indeed, quantitative proteomics reveal that elementary bodies contain an abundance of proteins that are required for central metabolism and glucose catabolism 15 , which might be used for a burst of metabolic activity on host cell entry and drive differentiation into reticulate bodies. During this differentiation, the crosslinked complexes are reduced, which provides the membrane fluidity that is required for replication 13 . Reticulate bodies specialize in nutrient acquisition and replication 7 ; they highly express proteins that are involved in the generation of ATP, protein synthesis and nutrient transport, such as V-type ATP synthases, ribosomal proteins and nucleotide transporters 15 . They probably rely on ATP scavenged from the host as an energy source, which indicates that the two developmental forms have distinct metabolic requirements 14 .
Binding to the host cell involves several bacterial ligands and host receptors 16 – 18 ( FIG. 1 ). On contact, pre-synthesized T3SS effectors are injected 15 and the elementary body is internalized into the inclusion. In a few hours (6–8 hours post-infection for C. trachomatis ), the transition to reticulate body takes place and early genes are transcribed 19 . Early effectors remodel the inclusion membrane, redirect exocytic vesicles to the inclusion and facilitate host–pathogen interactions 20 . Next, (~8–16 hours post-infection for C. trachomatis) mid-cycle genes are expressed, which include effectors that mediate nutrient acquisition and maintain the viability of the host cell. The bacteria divide by binary fission and the inclusion substantially expands. For some species, such as C. trachomatis , the infection of a single cell by several elementary bodies generates individual inclusions that fuse with each other by homotypic fusion 16 , 21 . At late stages (~24–72 hours post-infection for C. trachomatis ), reticulate bodies transition to elementary bodies in an asynchronous manner, which might be stimulated by their detachment from the inclusion membrane 11 . Late-cycle genes encode the outer membrane complex and the DNA binding histone H1-like and H2-like proteins, Hc1 and Hc2, which condense DNA and switch off the transcription of many genes 19 . Some late-cycle effectors that are generated at this time are packaged in progeny elementary bodies to be discharged in the next cycle of infection 11 , 15 .
This developmental cycle requires the finely tuned temporal expression of stage-specific factors, which involves alternative sigma factors, transcriptional activators and repressors, response regulators, small RNAs and regulators of DNA supercoiling 19 . The temporal classes of chlamydial genes mirror the stages of development. Although it is unclear how incoming elementary bodies become transcriptionally competent, early genes may be constitutively active or transcribed from supercoiling-insensitive promoters, as the level of supercoiling is low during the early stages of development 19 . Mid-cycle gene expression is driven by promoters that are responsive to increased DNA supercoiling, which is probably mediated by DNA gyrases that are expressed early during development 19 . The expression of mid-cycle genes may also be regulated by the atypical response regulator ChxR 19 , 22 . Late genes are regulated either by a stage-specific sigma factor (σ 28 ) 19 or through the relief of transcriptional repression of σ 66 -dependent promoters by the repressor early upstream open reading frame (EUO) 19 , 23 . Transcriptional regulation is also coupled to the T3SS 24 – 26 . Much of this insight comes from in vitro studies; advances in chlamydial genetics ( BOX 2 ) will enable the analysis of current models in vivo .
Box 2 | Advances in the genetic manipulation of Chlamydia spp.
After intensive efforts that have spanned decades, the genetic manipulation of chlamydiae has finally been successful. In retrospect, nature has provided numerous clues that genetic exchange with non-self DNA is limited in chlamydiae. The elementary body is relatively impermeable, whereas the reticulate body, which readily exchanges DNA, is separated from the external environment 13 . Chlamydiae lack genes that encode restriction and modification enzymes and harbour few, if any, vestiges of horizontal gene transfer, which is consistent with a lack of genetic exchange with other organisms 8 . Although chlamydial phages have been identified, they have not proven useful for the transduction of foreign DNA thus far 154 . Early attempts to transform Chlamydia spp. with shuttle vectors based on the Chlamydia `cryptic' plasmid resulted in only transient transformation 155 , possibly owing to the truncation of a gene that is essential for the maintenance of the plasmid 146 . Several recent breakthroughs have changed the landscape. First, gene replacement at the single rRNA locus in Chlamydia psittaci was achieved using classic allelic exchange combined with the elegant use of rRNA-specific drug-resistance markers 156 . This was a remarkable feat considering the inherent inefficiencies of a procedure that requires both transformation and homologous recombination. Second, a more efficient transformation protocol, using calcium chloride treatment of elementary bodies, was used to transform Chlamydia trachomatis with a shuttle vector that was derived from the naturally occurring C. trachomatis plasmid 157 . This shuttle vector also expresses the β-lactamase gene, which enables the selection of stable transformants with penicillin 157 . There is now an increasing number of shuttle vectors that enable the plasmid-based expression of genes that are fused to epitope tags, fluorescent proteins or secretion system reporters for the identification of effectors that are either under the control of inducible or native promoters 75 , 158 – 162 . Third, conventional chemical mutagenesis combined with whole-genome sequencing was used to construct a library of mapped C. trachomatis mutants 45 , 81 , 98 , 163 . By taking advantage of natural intra-inclusion genetic exchange between reticulate bodies 164 , mutants can be backcrossed with the parental strain to link genotype and phenotype. This approach, together with libraries that are generated by targeted mutagenesis 165 , will be useful for forward genetic screens and for identifying essential genes. Finally, several new strategies for creating targeted gene knockouts, including TILLING (targeting induced local lesions in genomes) and type II intron-mediated gene insertion, have been successfully used in Chlamydia spp. 166 , 167 . These recent successes will enable investigators to link genotypes with phenotypes and pave the way to test Koch's postulates with this obligate intracellular bacterium.
The developmental cycle can be reversibly arrested by environmental factors and stresses, such as nutrient deprivation, exposure to host cytokines and antibiotics that target cell wall synthesis 27 ( FIG. 1 ). Under these conditions, reticulate bodies transition to aberrantly enlarged, non-dividing `persistent' forms. Persistence may represent a stealthy approach to evade the immune system of the host. Although persistence might contribute to chronic inflammation and scarring, hallmarks of chlamydial disease, it remains controversial as to whether persistence occurs in vivo 3 .
Binding and invasion
The adhesion and invasion of Chlamydia spp. relies on numerous host and bacterial factors 16 – 18 ( FIGS 1 , 2a ). The diversity in binding and internalization mechanisms between species probably contributes to differences in tropism for specific hosts and tissues. The adhesion of C. trachomatis , C. pneumoniae and C. muridarum is a two-step process that is mediated by a low-affinity interaction with heparan sulfate proteoglycans (HSPGs) followed by high-affinity binding to host cell receptors 17 . OmcB (also known as CT443) from C. trachomatis L1 or C. pneumoniae mediates attachment to HSPGs 17 . The level and position of sulfation in HSPGs have important roles in the binding of C. muridarum and C. trachomatis L2 ( REFS 17 , 28 , 29 ) and may contribute to tissue tropism. Other adhesins include lipopolysaccharide (LPS) in C. trachomatis , which is proposed to bind to the cystic fibrosis transmembrane conductance regulator 17 , 30 , major outer membrane protein (MOMP; also known as CT681), which binds to the mannose receptor and the mannose 6-phosphate receptor 18 , and CT017 (also known as Ctad1) in C. trachomatis , which binds to β1 integrin 31 . The polymorphic membrane protein (Pmp) family in C. trachomatis and C. pneumoniae also mediates adhesion 32 . Pmp21 (also known as Cpn0963) binds to the epidermal growth factor receptor (EGFR) and functions as both an adhesin and an invasin 32 , 33 .
Figure 2. Chlamydia –host interactions.
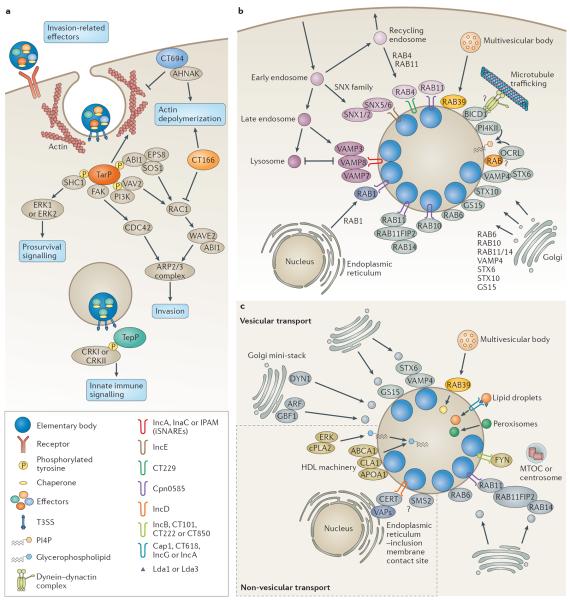
a | Elementary bodies contain pre-synthesized type III secretion system (T3SS) effectors along with their respective chaperones. On contact with host cells, invasion-related effectors are injected through the T3SS to induce cytoskeletal rearrangements and host signalling. In Chlamydia trachomatis , translocated actin-recruiting phosphoprotein (TarP), CT166 and CT694 are secreted first followed by TepP. TarP and TepP are tyrosine phosphorylated by host kinases. Phosphorylated TarP interacts with SRC homology 2 domain-containing transforming protein C1 (SHC1) to activate extracellular signal-regulated kinase 1 (ERK1; also known as MAPK3) and ERK2 (also known as MAPK1) for pro-survival signalling, whereas other phosphorylated TarP residues mediate interactions with two RAC guanine nucleotide exchange factors (GEFs), VAV2 and son of sevenless homologue 1 (SOS1). SOS1 is part of a multiprotein complex with ABL interactor 1 (ABI1) and epidermal growth factor receptor kinase substrate 8 (EPS8) in which ABI1 is thought to mediate the interaction of the complex with phosphorylated TarP, which leads to the activation of RAS-related C3 botulinum toxin substrate 1 (RAC1) and the host actin-related protein 2/3 (ARP2/3) complex. Phosphorylated TarP binds to the p85 subunit of phosphoinositide 3-kinase (PI3K), producing phosphatidylinositol-3,4,5-triphosphate PI(3,4,5)P 3 , which may activate VAV2. TarP also directly mediates the formation of actin filaments. TarP orthologues in Chlamydia caviae (which do not contain phosphorylation sites) bind to focal adhesion kinase (FAK) through a mammalian leucine–aspartic acid (LD2)-like motif and activate cell division control protein 42 (CDC42)-related actin assembly. CT694 contains a membrane-binding domain and interacts with the AHNAK protein, which links the membrane to the rearrangement of actin. CT166 glycosylates and inactivates RAC1. TarP activates the polymerization of actin, whereas CT694 and CT166 promote the depolymerization of actin. Phosphorylated TepP interacts with CRKI and CRKII to initiate innate immune signalling. b | The chlamydial inclusion is actively remodelled by host proteins and bacterial inclusion membrane proteins (Incs). Incs may regulate fusion with intracellular compartments and modulate membrane dynamics. Several RAB GTPases localize to the inclusion, including RAB4 and RAB11, which are recruited from recycling endosomes soon after entry by CT229 in C. trachomatis and Cpn0585 in Chlamydia pneumoniae , respectively. RAB11 is also recruited from the Golgi apparatus and binds to RAB11 family-interacting protein 2 (RAB11FIP2) to promote the recruitment of RAB14. RAB1 is recruited from the endoplasmic reticulum, whereas RAB6 and RAB10 relocalize from the Golgi apparatus. The RAB effector bicaudal-D homologue 1 (BICD1) may link the inclusion to dynein for transport along microtubules. The phosphatidylinositol-4-phosphate (PI4P)-producing enzymes OCRL1 and phosphatidylinositol-4-kinase type IIα (PI4KIIα) are recruited and may generate PI4P. Sorting nexin 5 (SNX5) and SNX6 are recruited from early endosomes by IncE to remodel the inclusion membrane and potentially inhibit retromer trafficking. RAB39a regulates the interaction between multivesicular bodies and the inclusion. Several soluble N -ethylmaleimide-sensitive factor attachment protein receptors (SNAREs) are recruited, including the Golgi-specific SNAREs vesicle-associated membrane protein 4 (VAMP4), syntaxin 6 (STX6) and GS15. In addition, the endocytic SNAREs, VAMP 3, VAMP 7 and VAMP 8, are recruited by IncA, InaC and inclusion protein acting on microtubules (IPAM), and are thought to act as inhibitory SNAREs (iSNAREs) to block the fusion with lysosomes. c | Chlamydia spp. interact with several subcellular compartments to acquire essential lipids. Sphingomyelin and cholesterol are incorporated into reticulate body membranes by intercepting vesicles from fragmented Golgi mini-stacks and multivesicular bodies. The trafficking of lipid-containing vesicles from Golgi mini-stacks is regulated by the GBF1-dependent activation of ADP-ribosylation factor (ARF) GTPases, by dynein heavy chain (DYN1) GTPase, and by Golgi-associated SNAREs and RABs. RAB39 mediates the interaction between the inclusion and multivesicular bodies. Lipid droplets and peroxisomes are translocated into the inclusion. Lipid droplets may be intercepted by Lda1 or Lda3, or by the Incs: Cap1, CT618, IncG and IncA. FYN kinase signalling from Inc microdomains that contain IncB, CT101, CT222 and CT850, contributes to lipid acquisition, possibly through the positioning of the inclusion at the microtubule-organizing centre (MTOC) or centrosome. Non-vesicular mechanisms of lipid acquisition involve the formation of endoplasmic reticulum–inclusion membrane contact sites (mediated by vesicle-associated membrane proteins (VAPs), the lipid transporter ceramide endoplasmic reticulum transport protein (CERT), and IncD), the recruitment of members of the high-density lipoprotein (HDL) machinery, and ERK signalling. The sphingomyelin biosynthetic enzyme, sphingomyelin synthase 2 (SMS2), may convert ceramide to sphingomyelin directly on the inclusion. ABCA1, ATP-binding cassette transporter 1; APOA1, apolipoprotein A1; CLA1, CD36 and LIMPII analagous 1; cPLA2, cytosolic phospholipase A2; WAVE2, Wiskott–Aldrich syndrome protein family member 2.
In addition to the β1 integrin subunit 31 and EGFR 33 , receptor tyrosine kinases (RTKs) contribute to binding, invasion and signalling during entry. C. trachomatis and C. muridarum interact with the fibroblast growth factor receptor (FGFR) and its ligand FGF as well as platelet derived growth factor receptor (PDGFR) 34 , 35 . C. trachomatis also binds to ephrin receptor A2 (EPHA2) to activate downstream signalling 36 , whereas apolipoprotein E4 may be a receptor for C. pneumoniae 37 . Finally, protein disulfide isomerase (PDI), a component of the oestrogen receptor complex, is implicated in the attachment and entry of many Chlamydia spp. 16 , 18 . PDI may also reduce the disulfide bonds in adhesins, host receptors and/or the T3SS 13 , 38 .
On contact with host cells, Chlamydia spp. induce actin remodelling, which promotes rapid internalization 16 , 39 ( FIG. 2a ). RHO-family GTPases, which are regulators of actin polymerization, are required for internalization, but the specific GTPase, or GTPases, differs between species 7 ; RAS-related C3 botulinum toxin substrate 1 (RAC1) is required for the entry of C. trachomatis , whereas cell division control protein 42 (CDC42) and ADP-ribosylation factor 6 (ARF6) contribute to the entry of Chlamydia caviae 18 . The activation of RAC1 results in the recruitment of the actin regulators, Wiskott–Aldrich syndrome protein family member 2 (WAVE2; also known as WASF2), ABL interactor 1 (ABI1), actin-related protein 2 (ARP2) and ARP3, which are necessary for the reorganization of actin 18 . Actin polymerization is accompanied by extensive membrane remodelling 16 , 39 , 40 , which is driven by several host factors, including caveolin, clathrin and cholesterol-rich microdomains 7 , 41 .
Pre-packaged effectors are injected through the T3SS to induce cytoskeletal rearrangements that promote invasion and activate host signalling 42 ( FIG. 2a ). Translocated actin-recruiting phosphoprotein (TarP; also known as CT456) is a multidomain protein that nucleates and bundles actin through its own globular actin (G-actin) and filamentous actin (F-actin) domains and is thought to synergize with the host ARP2/3 complex 16 , 43 . In addition, TarP in C. caviae contains a mammalian leucine–aspartic acid (LD2)-like motif that subverts focal adhesion kinase (FAK) signalling 44 . Some TarP orthologues contain an amino-terminal domain with 1–9 tyrosine-containing repeats, which are phosphorylated by ABL and SRC-family tyrosine kinases (SFKs) 16 . These phosphorylated residues participate in signalling for RAC-mediated actin rearrangements through VAV2 and son of sevenless homologue 1 (SOS1) and promote host cell survival through SRC homology 2 domain-containing transforming protein C1 (SHC1) 16 , 18 . TepP (also known as CT875), another T3SS effector that is phosphorylated by host tyrosine kinases, recruits the eukaryotic adaptor proteins CRKI and CRKII to the inclusion 45 . Both TarP and TepP, together with CT694 and CT695, use the chaperone Slc1 (also known as CT043) for secretion. The differential binding of TarP and TepP to Slc1 may explain the order of effector secretion, with TarP being secreted before TepP 45 , 46 . The T3SS effector CT694 is a multidomain protein that includes a membrane localization and an actin-binding AHNAK domain, which is thought to disrupt actin dynamics by interacting with the actin-binding protein AHNAK 11 , 47 . C. psittaci contains a weak orthologue of CT694, secreted inner nuclear membrane protein in Chlamydia (SINC; also known as G5Q_0070), which targets a conserved component of the nuclear `lamina' ( REF. 48 ) and may reflect pathogenic diversity between C. trachomatis and C. psittaci . The cytotoxin CT166, which resides in the plasticity zone of C. trachomatis genital strains, C. muridarum and C. caviae , inactivates the RHO GTPase RAC1 by glycosylation, which may reverse actin polymerization after entry 7 , 11 .
Establishing an intracellular niche
Migration to the microtubule organizing centre.
Nascent inclusions of some Chlamydia spp., including C. trachomatis and C. pneumoniae , are transported along microtubules to the microtubule-organizing centre (MTOC), which requires microtubule polymerization, the motor protein dynein and SFKs 16 , 49 ( FIG. 1 ). This facilitates interactions with nutrient-rich compartments and homotypic fusion 50 . Although some components of the dynactin complex are recruited, transport along micro tubules does not require p50 dynamitin 16 , 49 , which usually links cargo to microtubules. This suggests that bacterial effectors mimic the cargo-binding activity and possibly tether the inclusion to dynein and/or centrosomes. Four inclusion membrane proteins (Incs) in C. trachomatis (IncB (also known as CT232), CT101, CT222 and CT850) reside in cholesterol-rich microdomains at the point of centrosome–inclusion contact and colocalize with active SFKs 16 , 49 , which makes it likely that these Incs participate in transport. CT850 from C. trachomatis can directly bind to dynein light chain 1 (DYNLT1) to promote the positioning of the inclusion at the MTOC 51 . IncB from C. psittaci binds to Snapin, a protein that associates with host SNARE proteins (soluble N -ethylmaleimide-sensitive factor attachment protein receptor proteins) 52 . As Snapin can bind to both IncB and to dynein, it is possible that IncB–Snapin interactions connect the inclusion to the dynein motor complex for transport.
Regulation of fusion and membrane dynamics
The intracellular survival of Chlamydia spp. depends on the ability of the inclusion to inhibit fusion with some compartments (for example, with lysosomes) while promoting fusion with others (for example, with nutrient-rich exocytic vesicles). Chlamydia spp. achieve selective fusion by recruiting specific members of at least three families of fusion regulators ( FIG. 2b ): RAB GTPases and their effectors, phosphoinositide lipid kinases and SNARE proteins.
RAB GTPases are master regulators of vesicle fusion and particular RABs are recruited to inclusions, some of them in a species-specific manner 53 . RAB1, RAB4, RAB11 and RAB14 were recruited to all of the species tested, whereas RAB6 was recruited only to C. trachomatis and RAB10 was recruited only to C. pneumoniae . RAB4 and RAB11 mediate the interactions of the inclusion with the transferrin slow-recycling pathway to acquire iron 7 and may also contribute to transport along microtubules 53 . RAB6, RAB11 and RAB14 facilitate lipid acquisition from the Golgi apparatus 53 , whereas RAB39 participates in the delivery of lipids from multivesicular bodies 54 . The recruitment of RAB proteins is probably driven by species-specific Inc–RAB interactions. CT229 from C. trachomatis binds to RAB4, whereas Cpn0585 from C. pneumoniae binds to RAB1, RAB10 and RAB11 ( REF. 53 ). The RAB11 effector, RAB11 family-interacting protein 2 (RAB11FIP2), also localizes to the inclusion and, together with RAB11, promotes the recruitment of RAB14 ( REF. 55 ). Bicaudal-D homologue 1 (BICD1), a RAB6-interacting protein, is recruited to the inclusions of C. trachomatis L2 independently of RAB6, which reveals direct serovar-specific interactions 53 . Although the role of BICD1 is unclear, it may also contribute to transport to the MTOC, as this RAB effector family has been reported to link cargo to dynein 56 . RABs also promote vesicle fusion by recruiting lipid kinases, such as the inositol polyphosphate 5-phosphatase OCRL1 (also known as Lowe oculocerebrorenal syndrome protein), a Golgi-localized enzyme that produces the Golgi-specific lipid phosphatidylinositol-4-phosphate (PI4P) 53 . Another enzyme that produces PI4P, phosphatidylinositol-4-kinase type IIα (PI4KIIα), is also recruited to the inclusion. The enrichment of PI4P might disguise the inclusion as a specialized compartment of the Golgi apparatus 57 .
In addition, Chlamydia spp. control vesicle fusion by interacting with SNARE proteins ( FIG. 2b ). These include the trans -Golgi SNARE proteins syntaxin 6 (STX6) 58 , 59 and STX10 ( REF. 60 ), vesicle-associated membrane protein 4 (VAMP4), a STX6 binding partner 61 , and GS15 (also known as BET1L) 62 , which regulates the acquisition of nutrients from the Golgi exocytic pathway. The recruitment of STX6 requires a Golgi-targeting signal (YGRL) and VAMP4 ( REFS 59 , 61 ). In an example of molecular mimicry, at least three Incs contain SNARE-like motifs (IncA (also known as CT119), InaC (also known as CT813) and inclusion protein acting on microtubules (IPAM; also known as CT223)), which act as inhibitory SNARE proteins to limit fusion with compartments that contain VAMP3, VAMP7 or VAMP8 ( REFS 49 , 63 , 64 ). In addition, the dimerization of SNARE-like domains facilitates homotypic fusion 63 – 65 . Although the function of homotypic fusion is unclear, naturally occurring non-fusogenic strains of C. trachomatis produce fewer infectious progeny and milder infections 66 .
Finally, C. trachomatis recruits members of the sorting nexin (SNX) family 67 , 68 , which are involved in trafficking from the endosome to the Golgi apparatus 69 . The recruitment of SNX5 and SNX6 is mediated by a direct interaction with IncE (also known as CT116) 67 and may consequently disrupt this host trafficking pathway to promote bacterial growth.
Nutrient acquisition
Chlamydiae scavenge nutrients from various sources, for example, from the lysosomal-mediated degradation of proteins 7 , using various transporters 53 , 70 . Although chlamydiae can synthesize common bacterial lipids, the membrane of C. trachomatis contains eukaryotic lipids, including phosphatidylcholine, phosphatidylinositol, sphingomyelin and cholesterol 21 . These lipids are required for replication, homotypic fusion, growth and stability of the inclusion membrane, reactivation from persistence, and reticulate body to elementary body re-differentiation 21 , 71 . As Chlamydia spp. lack the required biosynthetic enzymes 8 , they have evolved sophisticated mechanisms to acquire lipids 21 that involve both vesicular and non-vesicular pathways ( FIG. 2c ). Sphingomyelin and cholesterol are acquired from the Golgi apparatus and multivesicular bodies 7 . Host proteins that are implicated in this vesicle-dependent acquisition include ARF GTPases 57 , 72 , 73 , the ARF guanine nucleotide exchange factor GBF1 ( REFS 72 , 73 ), RAB GTPases (specifically, RAB6, RAB11, RAB14 and RAB39) 53 , 54 , RAB11FIP2 ( REF. 55 ), VAMP4 ( REF. 61 ), dynamin 71 and FYN kinase 21 . Non-vesicular mechanisms involve lipid transporters, including the ceramide endoplasmic reticulum transport protein (CERT; also known as COL4α3BP) 73 , 74 , which directly binds to IncD (also known as CT115) 74 , 75 , and members of the high-density lipoprotein (HDL) biogenesis machinery, which deliver host phosphatidylcholine 76 . The acquisition of glycerophospholipids requires the activation of phospholipase A2 and mitogen-activated protein kinase (MAPK; also known as ERK) 21 . Sphingomyelin synthase 2 (SMS2; also known as SGMS2) is recruited to the inclusion, where it probably converts ceramide to sphingomyelin 73 . C. trachomatis also scavenges saturated fatty acids for de novo membrane synthesis 77 and can produce a unique phospholipid species through its own fatty acid and phospholipid biosynthetic machinery 78 . In fact, bacterial type II fatty acid synthesis is essential for the proliferation of chlamydiae 79 .
Golgi fragmentation
During the mid-cycle stages of infection with C. trachomatis , the Golgi apparatus is fragmented into mini-stacks that surround the inclusion, which is thought to increase the delivery of lipids 80 . Several host proteins have been implicated in this process, including RAB6 and RAB11, ARF GTPases, dynamin and inflammatory caspases 49 , 71 , 81 . At least one bacterial factor, InaC, is required for the redistribution of the fragmented Golgi apparatus, possibly through the action of ARF GTPases and 14-3-3 proteins 81 . Fragmentation requires remodelling of stable detyrosinated microtubules, which may function as mechanical anchors for the Golgi stacks at the surface of the inclusion 82 . The evaluation of the role of dynamin revealed that fragmentation is dispensable for the growth of C. trachomatis and for lipid uptake 71 . Similarly, the trafficking of sphingolipids in a mutant that is deficient in InaC is normal, which indicates that the acquisition of lipids from the Golgi apparatus does not require fragmentation 81 . Additional studies will be required to establish the role of Golgi fragmentation.

Interactions of the inclusion with other organelles
Chlamydia spp. establish close contact with numerous other organelles ( FIG. 2b ). Although many organelles and markers have been visualized inside the inclusion, some of these observations may have been exaggerated by fixation-induced translocation 83 . Lipid droplets 49 and peroxisomes 84 translocate into the lumen of the C. trachomatis inclusion and are a possible source of triacylglycerides and metabolic enzymes, respectively. Indeed, proteomic analysis of cells infected with C. trachomatis revealed an increase in lipid droplet content and an enrichment of proteins that are involved in lipid metabolism and biosynthesis, including long-chain-fatty-acid-CoA ligase 3 (ACSL3) and lysophosphatidylcholine acyltransferase 1 (LPCAT1) 85 . Interestingly, these proteins can also be found on, or in, the inclusion 86 . Some proteins that are associated with lipid droplets or peroxisomes may affect bacterial processes. For example, the human acyl-CoA carrier, acyl-CoA-binding domain-containing protein 6 (ACBD6), modulates the bacterial acyltransferase activity of CT775 in C. trachomatis and the formation of phophatidylcholine 86 , 87 . Whereas the mechanism of peroxisome uptake is unclear, the capture of lipid droplets may involve the chlamydial proteins Lda1, Lda3, Cap1 (also known as CT529) and CT618, as these proteins associate with lipid droplets when ectopically expressed in host cells 49 , 85 .
Mitochondria closely associate with the inclusion, and depletion of the translocase of the inner membrane–translocase of the outer membrane (TIM–TOM) complex, which imports mitochondrial proteins, disrupts infection with C. caviae and C. trachomatis 49 , 71 . The consequence of this interaction is unclear; however, it is possible that Chlamydia spp. acquire energy metabolites or dampen pro-apoptotic signals.
Finally, the inclusion membrane establishes close contact with the smooth endoplasmic reticulum 88 . These contact sites are enriched in host proteins that are usually found at endoplasmic reticulum–Golgi contact sites (CERT and its binding partners, and vesicle-associated membrane proteins (VAPs)) 73 , 74 and at endoplasmic reticulum–plasma membrane contact sites (the calcium sensor stromal interaction molecule 1 (STIM1)) 89 , and at least one bacterial protein, IncD in C. trachomatis 74 . This close contact may facilitate lipid transport 73 , 74 and the construction of signalling platforms 89 . Membrane contact sites participate in stimulator of interferon genes (STING)-dependent pathogen sensing 90 and could also modulate the endoplasmic reticulum stress response 91 , 92 . In addition, the inclusion is also juxtaposed to the rough endoplasmic reticulum, forming a `pathogen synapse' ( REF. 93 ). T3SS effectors may be specifically injected into the rough endoplasmic reticulum and/or the rough endoplasmic reticulum may help fold T3SS effectors 40 .
Stabilizing the inclusion
The inclusion membrane seems to be very fragile, as attempts to purify inclusions were only recently successful 68 . Consistent with this notion, the growing inclusion becomes encased in F-actin and intermediate filaments that form a dynamic scaffold, which provides structural stability and limits the access of bacterial products to the host cytosol 7 , 49 . The recruitment and assembly of F-actin involves RHO-family GTPases 49 , septins 94 , EGFR signalling 95 and at least one bacterial effector, InaC 81 . Microtubules are also actively reorganized around the inclusion by IPAM in C. trachomatis , which hijacks a centrosome protein, the centrosomal protein of 170 kDa (CEP170) 96 . This interaction initiates the organization of microtubules at the inclusion surface, which leads to the formation of a microtubule superstructure that is necessary for preserving membrane integrity 96 , 49 .
Exiting the host cell
The release of elementary bodies involves two mutually exclusive mechanisms: host cell lysis or the extrusion of the inclusion, which is a process that resembles exocytosis 97 ( FIG. 1 ). Lytic exit results in the death of the host cell and involves the permeabilization of the inclusion membrane, followed by permeabilization of the nuclear membrane, and lastly calcium-dependent lysis of the plasma membrane 97 . Live-cell imaging of a mutant that is deficient in a T2SS effector, Chlamydia protease-like activity factor (CPAF; also known as CT858), suggests that CPAF may have roles in dismantling the host cell and preparing elementary bodies for exit 98 . This report inferred that cytosolic CPAF was active only following lysis of the inclusion 98 , whereas other data suggest that the translocation of CPAF to the cytosol occurs before lysis and correlates with CPAF activity 99 . Further research is required to resolve when this T2SS effector reaches the cytosol of host cells.
By contrast, the extrusion pathway leaves the host cell intact and involves membrane pinching followed by expulsion of the inclusion. This process requires actin polymerization, the RHOA GTPase, neural Wiskott–Aldrich Syndrome protein (N-WASP), myosin II 97 and components of the myosin phosphatase pathway, such as myosin phosphatase-targeting subunit 1 (MYPT1; also known as PPP1R12A), which binds to the early transcribed Inc effector CT228 in C. trachomatis 100 . Some septin family members (septin 2, septin 9, septin 11 and possibly septin 7) have been implicated in egress of the inclusion, possibly through the recruitment or stabilization of F-actin 94 . In fact, the recruitment of actin may be required for the extrusion of the inclusion 101 . Extrusion prevents the release of inflammatory contents and protects elementary bodies from host immunity, and it could also contribute to persistence, as some bacteria remain in the host cell. To prevent re-infection, C. trachomatis induces the surface shedding of glycoprotein 96, resulting in the loss of PDI, which is involved in binding and invasion s 102 .
Modifying the host response
Controlling host cell survival and death.
Correctly timed cell death is important for intracellular pathogens, as premature host cell death can limit replication. Chlamydia spp. activate pro-survival pathways and inhibit apoptotic pathways 7 ( FIG. 3 ). At least three Chlamydia spp. bind to RTKs that in turn activate MEK–ERK and phosphoinositide 3-kinase (PI3K) survival pathways: MEK–ERK signalling is activated through the interaction of C. trachomatis and C. muridarum with FGFR 35 or through the binding of Pmp21 from C. pneumoniae to EGFR 33 . C. trachomatis also binds to EPHA2, which activates the PI3K pathway. These receptors are internalized with elementary bodies and, in the case of EPHA2, continue eliciting long-lasting survival signals, which are required for bacterial replication 36 . The upregulation of ERK also increases the level of EPHA2, which results in the activation of a feed-forward loop that is involved in host survival 36 . Finally, the Inc Cpn1027 from C. pneumoniae binds to members of the β-catenin–WNT pathway, cytoplasmic activation/proliferation-associated protein 2 (CAPRIN2) and glycogen synthase kinase 3β (GSK3β) 103 , which may enable β-catenin to activate the transcription of pro-survival genes.
Figure 3. Modulation of host cell survival and death.
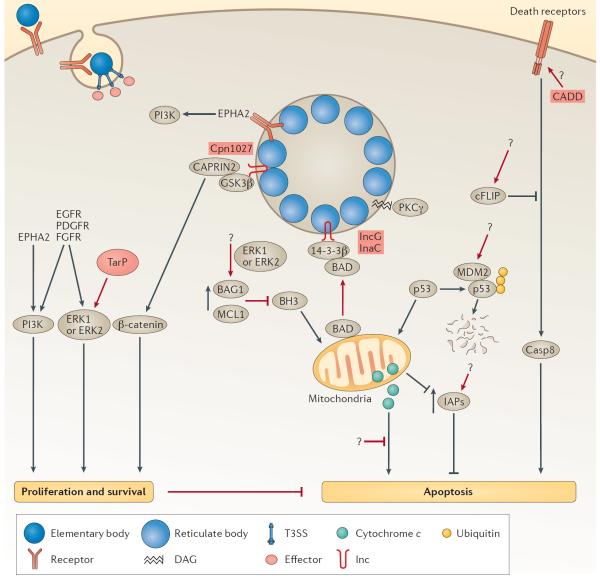
Chlamydial infection promotes host cell proliferation and survival through the activation of phosphoinositide 3-kinase (PI3K) and mitogen-activated protein kinase kinase (MAPKK, also known as MEK)–mitogen-activated protein kinase (MAPK, also known as ERK) signalling cascades by binding to receptor tyrosine kinases or through the secretion of the early effector translocated actin-recruiting phosphoprotein (TarP). Chlamydia pneumoniae sequesters cytoplasmic activation/proliferation-associated protein 2 (CAPRIN2) and glycogen synthase kinase 3β (GSK3β), members of the β-catenin destruction complex, to the inclusion membrane possibly through their interaction with Cpn1027, which leads to increased stabilization of β-catenin and transcriptional activation of survival genes by β-catenin. Infected host cells are resistant to various apoptotic stimuli and apoptosis is blocked both upstream and downstream of the permeabilization of the outer membrane of mitochondria by numerous mechanisms. The upregulation of the anti-apoptotic proteins BAG family molecular chaperone regulator 1 (BAG1) and myeloid leukaemia cell differentiation protein 1 (MCL1) inhibits the ability of BH3-only proteins to destabilize the mitochondrial membrane. The BH3-only protein BCL-2-associated agonist of cell death (BAD) is also sequestered at the inclusion membrane by binding to the host protein 14-3-3β. The degradation of p53 by MDM2-mediated ubiquitylation and sequestration of protein kinase Cγ (PKCγ) also prevents the depolarization of the mitochondrial membrane. Downstream of the release of cytochrome c , Chlamydia spp. are still able to prevent apoptosis through unknown mechanisms. Infection also leads to the upregulation of inhibitors of apoptosis (IAPs), which contributes to the anti-apoptotic phenotype. The chlamydial protein CADD is implicated in the modulation of apoptosis by binding to the death domains of tumour necrosis factor (TNF) family receptors. C. trachomatis can block the activation of caspase 8 through the regulator cellular FLICE-like inhibitory protein (cFLIP) by an unknown mechanism. The text in the red boxes denotes the individual steps in which Chlamydia spp. are proposed to modulate host cell function and the bacterial effector that is involved, if it is known. Casp8, caspase 8; DAG, diacylglycerol; EGFR, epidermal growth factor receptor; EPHA2, ephrin receptor A2; ERK, extracellular signal-regulated kinase; FGFR, fibroblast growth factor receptor; Inc, inclusion membrane protein; PDGFR, platelet derived growth factor receptor.
Cells that are infected with Chlamydia spp. show resistance to the stimuli of intrinsic and extrinsic apoptosis 104 ( FIG. 3 ). Resistance to apoptosis is cell-autonomous and requires bacterial protein synthesis 104 . Chlamydia spp. can block intrinsic apoptosis through numerous mechanisms including the MDM2-mediated ubiquitylation and proteasomal degradation of the tumour suppressor p53 ( REFS 105 , 106 ), the sequestration of pro-apoptotic protein kinase Cδ (PKCδ) or BCL-2-associated agonist of cell death (BAD) to the inclusion membrane through diacylglcerol or 14-3-3β-binding Incs, respectively 49 , 81 , and the upregulation or stabilization of anti-apoptotic proteins including BAG family molecular chaperone regulator 1 (BAG1), myeloid leukaemia cell differentiation protein 1 (MCL1) or cIAP2 (also known as BIRC3) 7 , 107 . C. trachomatis inhibits extrinsic apoptosis by blocking the activation of caspase 8 through the master regulator cellular FLICE-like inhibitory protein (cFLIP; also known as caspase 8-inhibitory protein) 108 . Proteomic analysis of intact C. trachomatis inclusions 68 and Inc binding partners 67 reveals additional host proteins and effectors that may regulate host cell survival.
Modulating the host cell cycle
Studies using immortalized cell lines reveal that infection with Chlamydia spp. modulates host progression through the cell cycle using several mechanisms 49 . Although these studies provide important insights, their physiological relevance should be viewed with caution as Chlamydia spp. replicate in terminally differentiated non-cycling cells in vivo .
Cell lines that are infected with C. trachomatis progress slower through the cell cycle, which was originally attributed to the degradation of cyclin B1; however, the observed degradation was probably an artefact of sample preparation 109 . The early expressed cytotoxin CT166 in C. trachomatis has also been implicated in slowing the progression of the cell cycle, as ectopic expression of CT166 delays G 1 -phase to S-phase transition by inhibiting MEK–ERK and PI3K signalling 110 . The mid-cycle effector CT847 in C. trachomatis interacts with host GRB2-related adapter protein 2 (GRAP2) and GRAP2 and cyclin-D-interacting protein (GCIP; also known as CCNDBP1); however, whether this interaction regulates progression through the cell cycle during infection is unclear 49 . Chlamydia spp. induce early mitotic exit by bypassing the spindle assembly checkpoint 111 . C. pneumoniae may mediate the arrest of the cell cycle through the T3SS effector, CopN, which has been reported to bind to microtubules and perturb their assembly in vitro ; however, whether this occurs during infection is unclear 112 , 113 . It is likely that Chlamydia spp. finely tune cell cycle progression to maximize nutrient acquisition at specific stages of development 114 .
C. trachomatis disrupts both centrosome duplication and clustering, but through independent mechanisms. CPAF is required for the induction of centrosome amplification, potentially through the degradation of one or more proteins that control centrosome duplication 111 . C. trachomatis also prevents centrosome clustering during mitosis in a CPAF-independent manner, presumably through the Inc-mediated tethering of centrosomes to the inclusion 51 , 111 , 115 , 116 . The cumulative effect of centro some amplification, early mitotic exit and errors in centrosome-positioning, halts cytokinesis, which results in multinucleated cells 111 , 114 . Multinucleation increases the contents of the Golgi apparatus, which enables Chlamydia spp. to easily acquire Golgi-derived lipids 114 . Other effectors that are implicated in multinucleation include IPAM, CT224, CT225 and CT166, as the ectopic expression of these proteins in uninfected cells blocks cytokinesis 49 , 110 , although their mechanism of action is unclear. In the case of IPAM, it will be interesting to determine whether the regulation of cytokinesis is mediated through binding to VAMPs and/or CEP170 and whether these observations represent three independent functions of IPAM.
Although infection induces double-strand breaks in the host DNA, chlamydiae dampen the host DNA-damage response 117 . C. trachomatis inhibits the binding of the DNA-damage response proteins MRE11, ataxia telangiectasia mutated (ATM) and p53-binding protein 1 (53BP1) to double-strand breaks 117 , and blocks the arrest of the cell cycle through the degradation of p53 ( REF. 106 ). By perturbing cell survival pathways, the regulation of the cell cycle, the repair of DNA damage, and centrosome duplication and positioning, chlamydiae favour malignant transformation. As the loss of the tumour suppressor p53 can produce extra centrosomes 118 , it will be interesting to determine whether the degradation of p53 is driving this process. Furthermore, Chlamydia spp. induce anchorage- independent growth in mouse fibroblasts, a phenotype that correlates closely with tumorigenicity 119 . These phenotypes seem to be relevant in vivo as infection of the mouse cervix increases cervical cell proliferation, signs of cervical dysplasia and chromosome abnormalities 119 . Even eukaryotic cells that are cleared of Chlamydia spp. show increased resistance to apoptosis and decreased responsiveness to p53 signalling 120 , which may increase sensitivity to malignant transformation.
Immune recognition and subversion of immunity
Epithelial cells recognize chlamydial antigens through cell surface receptors, endosomal receptors and cytosolic innate immune sensors ( FIG. 4 ). Activation of these receptors triggers the release of pro-inflammatory cytokines and chemokines, which recruit inflammatory cells 7 , 121 . However, this inflammatory response, which is required for bacterial clearance, is also responsible for immunopathology, such as tissue damage and scarring 6 .
Figure 4. Modulation of the innate immune response.
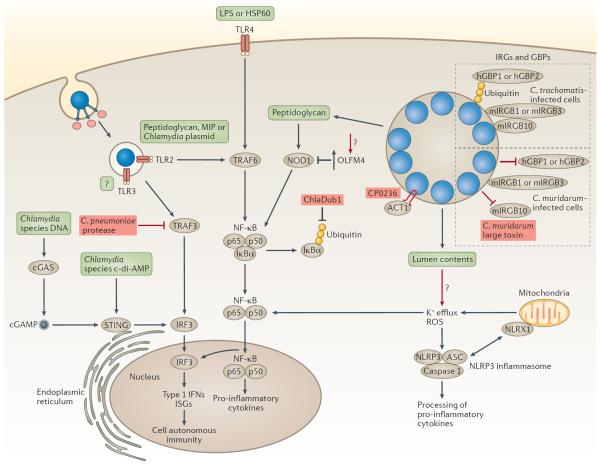
The recognition of chlamydial infection by pattern-recognition receptors leads to cell-autonomous immunity and the production of pro-inflammatory cytokines. Chlamydial antigens (green boxes) can be recognized by cell surface, endosomal or cytosolic pathogen sensors. Toll-like receptor 4 (TLR4) recognizes lipopolysaccharide (LPS) or the 60 kDa heat shock protein (HSP60), whereas TLR2 recognizes peptidoglycan, macrophage inhibitory protein (MIP) and/or plasmid-regulated ligands. Both TLR2 and TLR4 signalling require the adaptors myeloid differentiation primary response protein 88 (MYD88) and tumour necrosis factor (TNF) receptor-associated factor 6 (TRAF6), and lead to the nuclear translocation of nuclear factor-κB (NF-κB) and the induction of innate immune responses. The activation of the cytosolic sensor STING (stimulator of interferon genes) by the bacterial second messenger cyclic di-AMP (c-di-AMP) or through the host secondary messenger cyclic GMP–AMP (cGAMP) leads to the phosphorylation of interferon regulatory factor 3 (IRF3), nuclear translocation and induction of type I interferon (IFN) genes (encoding IFNα and IFNβ) and IFN-stimulated genes (ISG). The peptidoglycan sensor nucleotide-binding oligomerization domain-containing 1 (NOD1) is activated during chlamydial infection and induces the production of pro-inflammatory cytokines through NF-κB signalling. The activation of the NOD-, LRR- and pyrin domain-containing 3 (NLRP3)–apoptosis-associated speck-like protein containing a CARD (ASC) inflammasome (NLRP3–ASC inflammasome) requires reactive oxygen species (ROS) and K + efflux. The production of ROS is amplified by the mitochondrial NOD-like receptor X1 (NLRX1), which augments the production of ROS, creating a feed-forward loop. Some innate immune molecules that recognize vacuolar pathogens, such as human guanylate-binding protein 1 (hGBP1) and hGBP2, mouse immunity-related GTPase family M protein 1 (mIRGM1) and mIRGM3, and mouse IRGB10 (mIRGB10), localize to Chlamydia trachomatis inclusions and promote bacterial clearance. Chlamydia muridarum prevents the recognition of inclusions by these receptors. Chlamydial virulence factors (depicted in red) can disrupt or augment the innate immune response. During infection with Chlamydia pneumoniae , an unknown protease cleaves TRAF3, which blocks the phosphorylation of IRF3 and the production of type 1 IFNs. The deubiquitinase Dub1 removes ubiquitin from NF-κB inhibitor-α (IκBα), which stabilizes the p65–p50–IκBα complex and prevents the nuclear translocation of NF-κB. The C. pneumonia protein CP0236 sequesters NF-κB activator 1 (ACT1) to the inclusion membrane. Chlamydial infection also leads to the upregulation of olfactomedin 4 (OLFM4), which potentially blocks NOD1-mediated signalling. cGAS, cGAMP synthase.
On binding, chlamydiae activate the Toll-like receptors (TLRs) — TLR2, TLR3 and TLR4 — to varying degrees, depending on the species and the infection model 6 , 7 , 121 , 122 . TLR2 can recognize peptidoglycan, although other ligands, such as macrophage inhibitory protein (MIP) or plasmid-regulated ligands, have been suggested 121 . TLR2 and the downstream TLR adaptor myeloid differentiation primary response protein 88 (MYD88) have been reported to localize on, and potentially signal from, the inclusion, and mouse models of infection with C. pneumoniae and C. trachomatis suggest key roles for TLR2 and MYD88 in bacterial recognition and clearance 121 , 123 . The recognition of chlamydial LPS and/or 60 kDa heat shock protein (HSP60) by TLR4 may also contribute to bacterial clearance, but the relative importance of TLR4 compared with TLR2 remains to be determined 121 , 123 . Chlamydial infection is also detected by the intracellular nucleotide sensors cyclic GMP–AMP (cGAMP) synthase (cGAS) and STING 124 . On binding DNA, cGAS catalyses the production of the cyclic di-nucleotide second messenger cGAMP, which activates STING, inducing the expression of type I interferons (IFNs) 125 . Chlamydia spp. also synthesize the secondary messenger cyclic di-AMP, which, on release into the cytoplasm of the host cell, activates STING independently of cGAS, and may contribute to the production of type I IFNs 90 . The intracellular peptidoglycan-binding molecule nucleotide-binding oligomerization domain-containing 1 (NOD1) is also activated in response to infection with C. pneumoniae , C. trachomatis and C. muridarum , which reveals that chlamydial peptidoglycan gains access to the host cytosol ( BOX 3 ). During chlamydial infection, caspase 1 is activated through the NOD-, LRR- and pyrin domain-containing 3 (NLRP3)-apoptosis-associated speck-like protein containing a CARD (ASC) inflammasome (NLRP3–ASC inflammasome) 121 . Activation of the NLRP3–ASC inflammasome requires K + efflux and the production of reactive oxygen species (ROS), which is amplified through the mitochondrial NOD-like receptor X1 (NLRX1) 126 . Intriguingly, activation of the inflammasome promotes infection under some conditions 121 , 127 , maybe owing to an increase in lipid acquisition or utilization 127 . Finally, maintaining the integrity of the inclusion membrane is probably another mechanism used to prevent the cytosolic sensing of bacterial components 121 .
Box 3 | Solving the chlamydial peptidoglycan `anomaly'.
Peptidoglycan forms a lattice-like sheet that surrounds the cytoplasmic membrane of bacterial cells, where it has crucial functions in binary fission and in maintaining structural strength against osmotic pressure 168 . It is expected that chlamydiae, similarly to other Gram-negative pathogens, have peptidoglycan in their cell walls. Chlamydiae encode functional peptidoglycan biosynthetic enzymes, are sensitive to β-lactam antibiotics that target peptidoglycan biosynthesis, and activate the host cytosolic sensor of peptidoglycan — nucleotide-binding oligomerization domain-containing 1 (NOD1) 168 . Despite these observations, peptidoglycan was not detectable using conventional methods; a dilemma that was termed the `chlamydial anomaly' ( REF. 168 ). Furthermore, chlamydiae lack FtsZ 8 , which, together with peptidoglycan, coordinates cell division and cell shape in nearly all other bacterial species 168 . Therefore, chlamydiae must use alternative strategies to coordinately orchestrate peptidoglycan synthesis and cell division. Several new studies have helped to resolve these discrepancies. First, an intact peptidoglycan-containing sacculus was extracted from the ancestral Protochlamydiae, and was found to contain a novel peptidoglycan modification 169 . Second, using a new technique that is based on the incorporation of click-chemistry-modified d -amino acid probes, newly assembled peptidoglycan was visualized at the septum of dividing reticulate bodies in a ring-like structure, which indicated that peptidoglycan may have a role in cell division 170 . Third, MreB, an actin homologue, was found to localize at the septum in a manner that was dependent on peptidoglycan precursors and its conserved regulator RodZ (also known as CT009) 171 – 173 , where it is thought to functionally compensate for the lack of FtsZ. Fourth, muramyl peptides and larger muropeptide fragments in Chlamydia trachomatis were isolated from fractionated infected cell lysates using a NOD-activated reporter cell line as a sensitive read-out 174 . Mass spectrometry analysis of these peptides provided the first structural confirmation of chlamydial peptidoglycan and demonstrated that chlamydiae can carry out transpeptidation and transglycosylation reactions 174 . Fifth, the amidase A homologue (AmiA) in Chlamydia pneumoniae was shown to use lipid II, the peptidoglycan building block comprised of N -acetylglucosamine (GlcNAc) and N -acetylmuramic acid (MurNAc)-pentapeptide, as a substrate 175 . AmiA is unusual in that it has dual enzymatic activity, functioning both as an amidase and as a penicillin-sensitive carboxypeptidase that is crucial for the complete biosynthesis of lipid II, the remodelling of peptidoglycan and for maintaining coordinated cell division 175 , 176 . Finally, C. pneumoniae and Waddlia chondrophila each encode a functional homologue of Escherichia coli NlpD, which localizes to the septum where it probably acts on peptide crosslinks 175 , 176 . Together, these studies provide strong evidence that chlamydiae express classical peptidoglycan, which is probably required for cell division.
Chlamydiae have evolved several mechanisms to manipulate immune responses, and under some conditions, prevent clearance. Chlamydial infection can mitigate the production of IFN or counteract downstream gene products that are involved in cell-autonomous immunity 121 . C. pneumoniae suppresses the production of IFNβ by degrading the signalling molecule tumour necrosis factor (TNF) receptor-associated factor 3 (TRAF3), possibly through the activity of a protease that is specific to C. pneumoniae 128 . C. pneumoniae also evades the production of nitric oxide (NO) by downregulating the transcription of inducible NO synthase (iNOS) through the induction of an alternative polyamine pathway 129 . C. trachomatis downregulates the expression of interferon- induced protein with tetratricopeptide repeats 1 (IFIT1) and IFIT2 through the T3SS effector TepP 45 . The production of IFNγ induces the expression of host indoleamine 2,3-dioxygenase (IDO), which depletes host cells of tryptophan, thereby inhibiting the replication of Chlamydia strains that are tryptophan auxotrophs 27 . Genital serovars encode a tryptophan synthase that enables the synthesis of tryptophan from indole that is provided by the local microbiota, thus evading this host response. IFNγ also induces the expression of immunity-related GTPases (IRGs) or guanylate binding proteins (GBPs) 49 . These dynamin-like molecules aid in the recognition of the inclusion, directly damage the inclusion and/or modulate the fusion of the inclusion with lysosomes. Defining the role of IRGs and GBPs in chlamydial infection has been complex, as the anti-chlamydial effects of IFNγ are specific to the host, the cell-type and the Chlamydia spp. Mouse cells recognize C. trachomatis inclusions through the IFN-inducible GTPases IRGM1, IRGM3 and IRGB10, but the recruitment of IRGB10 to the inclusions is prevented by an unknown mechanism 49 . Human GBP1 and GBP2 recognize and localize to C. trachomatis inclusions and contribute to the effects of IFNγ, possibly through the recruitment of the autophagic machinery 130 . Recent studies suggest that the ubiquitylation of the C. trachomatis inclusion is important for the recruitment of GBPs 131 , 132 , which enable the rapid activation of the inflammasome in infected macrophages 133 . This process may be important for distinguishing between self-vacuoles and Chlamydia -containing vacuoles 134 .
Chlamydiae use various strategies to evade or dampen nuclear factor-κB (NF-κB) transcription 7 , 16 . The T3SS effector ChlaDub1 (also known as CT868) deubiquitylates and stabilizes NF-κB inhibitor-α (IκBα) in the cytosol, whereas the C. pneumoniae Inc CP0236 binds to and sequesters NF-κB activator 1 (ACT1; also known as CIKS) to the inclusion membrane 135 , thereby blocking NF-κB signalling. Infection with C. trachomatis also upregulates olfactomedin 4 (OLFM4), a glycoprotein that may suppress the NOD1-mediated activation of NF-κB 136 . These redundant mechanisms highlight the importance of blocking NF-κB.
Alterations in the host cell transcriptome and proteome
Similar to other intracellular pathogens, Chlamydia spp. cause substantial changes in gene expression and protein production in the host, at the transcriptional, translational and post-translational levels. In addition to affecting transcriptional regulators, such as NF-κB 121 ( FIG. 4 ), Chlamydia spp. markedly alter histone post-translational modifications 117 , which can change the structure of chromatin and gene expression. The T3SS effector CT737 (also known as NUE) encodes a putative histone methyltransferase that associates with host chromatin during infection with C. trachomatis and may have a role in the observed global histone modification 137 . C. trachomatis also induces widespread changes in protein stability, and at least a subset of these altered proteins is required for replication 138 . Chlamydia spp. secrete T3SS effectors that modulate host ubiquitylation and protein stability, including the deubiquitinase Cpn0483 (also known as Chla OTU) produced by C. pneumoniae 139 and the deubiquitinases ChlaDub1 and ChlaDub2 (also known as CT867) produced by C. trachomatis 140 , 141 . Furthermore, Incs that are produced by C. trachomatis may interact with the host ubiquitylation machinery 67 , which reveals additional routes to target protein stability. Historically, CPAF was a strong candidate for reshaping the host proteome, as CPAF can cleave numerous and diverse substrates in vitro and its activity has been attributed to several phenotypes in vivo 109 , 142 . However, the interpretation of CPAF activity and its substrates is complicated by artefactual proteolysis during sample preparation 109 , 143 , which has triggered intense discussion 142 , 144 . Consistent with the results of Chen et al. 109 , a CPAF-null mutant reveals that this protease is not required for some of the phenotypes that were previously suggested, including Golgi fragmentation, the inhibition of NF-κB activation and resistance to apoptosis 98 , 145 . Future genetic studies will determine how Chlamydia spp. induce global changes in the host proteome in vivo and the role and physiologically relevant substrates of CPAF.
Conclusions and future directions
Understanding how Chlamydia spp. survive in the intracellular environment reveals the finely nuanced `arms race' between pathogens and their hosts. Despite its reduced genome size, this wily pathogen uses its arsenal of effectors to establish an intracellular niche and modulate the host immune response. New techniques in host and bacterial cell biology, proteomics and host genetics have provided vital insights into the molecular underpinnings of these events. Recent breakthroughs in chlamydial genetics 146 ( BOX 2 ) have opened the possibility of delineating gene functions in vivo , including the roles of specific bacterial adhesins and effectors, to test the current models of temporal gene expression, and to help resolve questions regarding the function of CPAF 98 , 99 , 144 . Further technological advances will eventually lead to efficient and versatile genome editing. Finally, recent improvements in cell culture 147 – 149 and animal models 3 will continue to increase our understanding of pathogenic processes, as these models more closely recapitulate the pathology of human genital tract infections, and will probably be useful in investigating the effects of clinically important co-infecting pathogens, such as HIV or herpes simplex virus. We are entering a new golden age in understanding the pathogenic mechanisms commandeered by this medically important and fascinating obligate intracellular pathogen.
Acknowledgements
The authors apologize to those colleagues in the field whose work could not be included owing to space constraints. The authors gratefully acknowledge financial support from the US National Institutes of Health (R01 AI073770, AI105561 and AI122747) to J.E., and from the University of California, San Francisco (UCSF)-Gladstone Institute for Virology and Immunology, and the Center for AIDS Research to C.E.
Variants that differ physiologically and/or biochemically from other strains of a particular species.
A subdivision of a species or subspecies that is distinguished by a characteristic set of antigens.
A system that exports autotransporters that are composed of a carboxy-terminal β-barrel translocator domain and an amino-terminal passenger domain that passes through the interior of the barrel to face the external environment.
A system that exports proteins across the bacterial inner membrane through the general secretory (Sec) pathway and across the outer membrane through secretin. In chlamydiae, T2SS effectors are secreted into the inclusion lumen, but can access the host cytosol through outer membrane vesicles.
A needle-like apparatus found in Gram-negative bacteria that delivers proteins, called effectors, across the inner and outer bacterial membranes and across eukaryotic membranes to the host membrane or the cytosol.
The fusion between cells or vesicles of the same type. For cells that are infected with some Chlamydia spp., several inclusions undergo fusion with each other to form one, or a few, larger inclusions.
Bacterial proteins that direct the binding of RNA polymerase to promoters, which enables the initiation of transcription.
Subunits of bacterial two-component signal transduction pathways that regulate output in response to an environmental stimulus.
A member of a diverse group of surface-localized, immunodominant proteins that are secreted by the type V secretion system (T5SS) of Chlamydia spp.
A member of the GTPase family of small GTPases that regulates vesicular transport, in which they function to recruit coat proteins that are necessary for the formation of vesicles.
A multisubunit complex found in eukaryotic cells that binds to the motor protein dynein and aids in the microtubule-based transport of vesicles.
Soluble N -ethylmaleimide-sensitive factor (NSF) attachment protein receptor proteins). Soluble NSF attachment proteins are a large superfamily of proteins that mediate vesicle fusion by pairing with each other on adjacent membranes through SNARE domains.
Small GTPases that localize to the cytosolic face of specific intracellular membranes, where they regulate intracellular trafficking and membrane fusion. Different RABs are specific for distinct subcellular compartments.
A family of enzymes that generates phosphorylated variants of phosphatidylinositols (secondary messengers), which are important for signalling and membrane remodelling. Organelles are, in part, identified by the specific lipid species that they contain.
A specialized set of late endosomes that contains internal vesicles formed by the inward budding of the outer endosomal membrane. MVBs are involved in protein sorting and are rich in lipids, including sphingolipids and cholesterol.
A large GTPase that is involved in the scission of newly formed vesicles from the cell surface, endosomes and the Golgi apparatus.
A cytosolic protein that mediates the non-vesicular transport of ceramide from the endoplasmic reticulum to the Golgi apparatus where it is converted to sphingomyelin.
A type of fatty acid synthesis in which the enzymes that catalyse each step in the synthesis pathway exist as distinct, individual proteins rather than as a multi-enzyme complex as in type I fatty acid synthesis.
A family of cysteine proteases that has essential roles in apoptosis, necrosis and inflammation.
A family of conserved regulatory molecules that usually binds to a phosphoserine or phosphothreonine residue found in functionally diverse signalling proteins in eukaryotic cells.
A signalling molecule that recognizes cytosolic cyclic dinucleotides and activates the production of type I interferons.
A stress pathway that is activated by perturbations in protein folding, lipid and steroid biosynthesis, and intracellular calcium stores.
A family of GTP-binding proteins that assembles into oligomeric complexes to form large filaments and rings that act as scaffolds and diffusion barriers
A type II secreted broad-spectrum protease produced by Chlamydia spp. that may have a role in cleaving host proteins on release into the host cell cytosol late in infection or extracellularly following the lysis of the host cell.
Members of the B cell lymphoma 2 (BCL-2) protein family, which are essential initiators of programmed cell death and are required for apoptosis that is induced by cytotoxic stimuli.
A eukaryotic signal transduction pathway that signals through the binding of the WNT protein ligand to a frizzled family cell surface receptor, which results in changes to gene transcription, cell polarity or intracellular calcium levels.
A programmed form of cell death that involves the degradation of cellular constituents by caspases that are activated through either the intrinsic (mitochondria-mediated) or extrinsic (death receptor-mediated) apoptotic pathways.
A eukaryotic tumour suppressor that maintains genome integrity by activating DNA repair, arresting the cell cycle or initiating apoptosis.
The physical process of cell division, which divides the cytoplasm of a parental cell into two daughter cells.
Transmembrane proteins that have a key role in the innate immune system by recognizing pathogen-associated molecular patterns (PAMPs), which are structurally conserved molecules derived from microorganisms.
A subgroup of interferon proteins produced as part of the innate immune response against intracellular pathogens.
A cytosolic pattern-recognition receptor that recognizes bacterial peptidoglycan.
A multiprotein complex, consisting of caspase1, NOD-, LRR- and PYD domain-containing protein 3 (NLRP3) and apoptosis-associated speck-like protein containing a CARD (ASC), that processes the pro-inflammatory cytokines interleukin-1β (IL-1β) and IL-18 into their mature forms.
The ability of a host cell to eliminate an invasive infectious agent, which relies on microbial proteins, specialized degradative compartments and programmed host cell death.
A protein complex that is translocated from the cytosol to the nucleus and regulates DNA transcription, cytokine production and cell survival in response to harmful cellular stimuli.
Competing interests statement The authors declare no competing interests.
- 1. Bachmann NL, Polkinghorne A, Timms P. Chlamydia genomics: providing novel insights into chlamydial biology. Trends Microbiol. 2014;22:464–472. doi: 10.1016/j.tim.2014.04.013. [ DOI ] [ PubMed ] [ Google Scholar ]
- 2. Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. 7th edn Vol. 2. Churchill Livingstone/Elsevier; 2010. [ Google Scholar ]
- 3. Rank RG. In: Intracellular Pathogens 1: Chlamydiales. Tan M, Bavoil PM, editors. Vol. 1. ASM Press; 2012. pp. 285–310. [ Google Scholar ]
- 4. Malhotra M, Sood S, Mukherjee A, Muralidhar S, Bala M. Genital Chlamydia trachomatis: an update. Indian J. Med. Res. 2013;138:303–316. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 5. de Vrieze NH, de Vries HJ. Lymphogranuloma venereum among men who have sex with men. An epidemiological and clinical review. Expert Rev. Anti Infect. Ther. 2014;12:697–704. doi: 10.1586/14787210.2014.901169. [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Murthy AK, Arulanandam BP, Zhong G. In: Intracellular Pathogens 1: Chlamydiales. Tan M, Bavoil PM, editors. Vol. 1. ASM Press; 2012. pp. 311–333. [ Google Scholar ]
- 7. Bastidas RJ, Elwell CA, Engel JN, Valdivia RH. Chlamydial intracellular survival strategies. Cold Spring Harb. Perspect. Med. 2013;3:a010256. doi: 10.1101/cshperspect.a010256. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 8. Stephens RS, et al. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science. 1998;282:754–759. doi: 10.1126/science.282.5389.754. [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. Myers GSA, Crabtree J, Creasy HH. In: Intracellular Pathogens 1: Chlamydiales. Tan M, Bavoil PM, editors. Vol. 1. ASM press; 2012. pp. 27–50. [ Google Scholar ]
- 10. Betts-Hampikian HJ, Fields KA. The chlamydial type III secretion mechanism: revealing cracks in a tough nut. Front. Microbiol. 2010;1:114. doi: 10.3389/fmicb.2010.00114. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 11. Fields KA. In: Intracellular Pathogens 1: Chlamydiales. Tan M, Bavoil PM, editors. Vol. 1. ASM press; 2012. pp. 192–216. [ Google Scholar ]
- 12. Lei L, et al. Reduced live organism recovery and lack of hydrosalpinx in mice infected with plasmid-free Chlamydia muridarum. Infect. Immun. 2014;82:983–992. doi: 10.1128/IAI.01543-13. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 13. Nelson DE. In: Intracellular Pathogens 1: Chlamydiales. Tan M, Bavoil PM, editors. Vol. 1. ASM Press; 2012. pp. 74–96. [ Google Scholar ]
- 14. Omsland A, Sixt BS, Horn M, Hackstadt T. Chlamydial metabolism revisited: interspecies metabolic variability and developmental stage-specific physiologic activities. FEMS Microbiol. Rev. 2014;38:779–801. doi: 10.1111/1574-6976.12059. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 15. Saka HA, et al. Quantitative proteomics reveals metabolic and pathogenic properties of Chlamydia trachomatis developmental forms. Mol. Microbiol. 2011;82:1185–1203. doi: 10.1111/j.1365-2958.2011.07877.x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 16. Hackstadt T. In: Intracellular Pathogens 1: Chlamydiales. Tan M, Bavoil PM, editors. Vol. 1. ASM press; 2012. pp. 126–148. [ Google Scholar ]
- 17. Hegemann JH, Moelleken K. In: Intracellular Pathogens 1: Chlamydiales. Tan M, Bavoil PM, editors. Vol. 1. ASM press; 2012. pp. 97–125. [ Google Scholar ]
- 18. Mehlitz A, Rudel T. Modulation of host signaling and cellular responses by Chlamydia. Cell Commun. Signal. 2013;11:90. doi: 10.1186/1478-811X-11-90. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 19. Tan M. In: Intracellular Pathogens 1: Chlamydiales. Tan M, Bavoil PM, editors. Vol. 1. ASM press; 2012. pp. 149–169. [ Google Scholar ]
- 20. Moore ER, Ouellette SP. Reconceptualizing the chlamydial inclusion as a pathogen-specified parasitic organelle: an expanded role for Inc proteins. Front. Cell. Infect. Microbiol. 2014;4:157. doi: 10.3389/fcimb.2014.00157. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 21. Elwell CA, Engel JN. Lipid acquisition by intracellular Chlamydiae. Cell. Microbiol. 2012;14:1010–1018. doi: 10.1111/j.1462-5822.2012.01794.x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 22. Barta ML, et al. Atypical response regulator ChxR from Chlamydia trachomatis is structurally poised for DNA binding. PLoS ONE. 2014;9:e91760. doi: 10.1371/journal.pone.0091760. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 23. Rosario CJ, Hanson BR, Tan M. The transcriptional repressor EUO regulates both subsets of Chlamydia late genes. Mol. Microbiol. 2014;94:888–897. doi: 10.1111/mmi.12804. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 24. Barta ML, Battaile KP, Lovell S, Hefty PS. Hypothetical protein CT398 (CdsZ) interacts with σ54 (RpoN)-holoenzyme and the type III secretion export apparatus in Chlamydia trachomatis. Protein Sci. 2015;24:1617–1632. doi: 10.1002/pro.2746. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 25. Hanson BR, Slepenkin A, Peterson EM, Tan M. Chlamydia trachomatis type III secretion proteins regulate transcription. J. Bacteriol. 2015;197:3238–3244. doi: 10.1128/JB.00379-15. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 26. Shen L, et al. Multipart chaperone–effector recognition in the type III secretion system of Chlamydia trachomatis. J. Biol. Chem. 2015;290:28141–28155. doi: 10.1074/jbc.M115.670232. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 27. Byrne GI, Beatty WL. In: Intracellular Pathogens 1: Chlamydiales. Tan M, Bavoil PM, editors. Vol. 1. ASM press; 2012. pp. 265–284. [ Google Scholar ]
- 28. Kim JH, et al. Endosulfatases SULF1 and SULF2 limit Chlamydia muridarum infection. Cell. Microbiol. 2013;15:1560–1571. doi: 10.1111/cmi.12133. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 29. Rosmarin DM, et al. Attachment of Chlamydia trachomatis L2 to host cells requires sulfation. Proc. Natl Acad. Sci. USA. 2012;109:10059–10064. doi: 10.1073/pnas.1120244109. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 30. Ajonuma LC, et al. CFTR is required for cellular entry and internalization of Chlamydia trachomatis. Cell Biol. Int. 2010;34:593–600. doi: 10.1042/CBI20090227. [ DOI ] [ PubMed ] [ Google Scholar ]
- 31. Stallmann S, Hegemann JH. The Chlamydia trachomatis Ctad1 invasin exploits the human integrin β1 receptor for host cell entry. Cell. Microbiol. 2015 doi: 10.1111/cmi.12549. http://dx.doi.org/10.1111/cmi.12549 . [ DOI ] [ PubMed ]
- 32. Becker E, Hegemann JH. All subtypes of the Pmp adhesin family are implicated in chlamydial virulence and show species-specific function. Microbiologyopen. 2014;3:544–556. doi: 10.1002/mbo3.186. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 33. Molleken K, Becker E, Hegemann JH. The Chlamydia pneumoniae invasin protein Pmp21 recruits the EGF receptor for host cell entry. PLoS Pathog. 2013;9:e1003325. doi: 10.1371/journal.ppat.1003325. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 34. Elwell CA, Ceesay A, Kim JH, Kalman D, Engel JN. RNA interference screen identifies Abl kinase and PDGFR signaling in Chlamydia trachomatis entry. PLoS Pathog. 2008;4:e1000021. doi: 10.1371/journal.ppat.1000021. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 35. Kim JH, Jiang S, Elwell CA, Engel JN. Chlamydia trachomatis co-opts the FGF2 signaling pathway to enhance infection. PLoS Pathog. 2011;7:e1002285. doi: 10.1371/journal.ppat.1002285. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 36. Subbarayal P, et al. EphrinA2 receptor (EphA2) is an invasion and intracellular signaling receptor for Chlamydia trachomatis. PLoS Pathog. 2015;11:e1004846. doi: 10.1371/journal.ppat.1004846. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 37. Gerard HC, Fomicheva E, Whittum-Hudson JA, Hudson AP. Apolipoprotein E4 enhances attachment of Chlamydophila (Chlamydia) pneumoniae elementary bodies to host cells. Microb. Pathog. 2008;44:279–285. doi: 10.1016/j.micpath.2007.10.002. [ DOI ] [ PubMed ] [ Google Scholar ]
- 38. Ferrell JC, Fields KA. A working model for the type III secretion mechanism in Chlamydia. Microbes Infect. 2015;18:84–92. doi: 10.1016/j.micinf.2015.10.006. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 39. Nans A, Saibil HR, Hayward RD. Pathogen–host reorganization during Chlamydia invasion revealed by cryo-electron tomography. Cell. Microbiol. 2014;16:1457–1472. doi: 10.1111/cmi.12310. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 40. Dumoux M, Nans A, Saibil HR, Hayward RD. Making connections: snapshots of chlamydial type III secretion systems in contact with host membranes. Curr. Opin. Microbiol. 2015;23:1–7. doi: 10.1016/j.mib.2014.09.019. [ DOI ] [ PubMed ] [ Google Scholar ]
- 41. Korhonen JT, et al. Chlamydia pneumoniae entry into epithelial cells by clathrin-independent endocytosis. Microb. Pathog. 2012;52:157–164. doi: 10.1016/j.micpath.2011.12.002. [ DOI ] [ PubMed ] [ Google Scholar ]
- 42. Dai W, Li Z. Conserved type III secretion system exerts important roles in Chlamydia trachomatis. Int. J. Clin. Exp. Pathol. 2014;7:5404–5414. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 43. Jiwani S, et al. Chlamydia trachomatis TarP harbors distinct G and F actin binding domains that bundle actin filaments. J. Bacteriol. 2013;195:708–716. doi: 10.1128/JB.01768-12. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 44. Thwaites T, et al. The Chlamydia effector TarP mimics the mammalian leucine–aspartic acid motif of paxillin to subvert the focal adhesion kinase during invasion. J. Biol. Chem. 2014;289:30426–30442. doi: 10.1074/jbc.M114.604876. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 45. Chen YS, et al. The Chlamydia trachomatis type III secretion chaperone Slc1 engages multiple early effectors, including TepP, a tyrosine-phosphorylated protein required for the recruitment of CrkI-II to nascent inclusions and innate immune signaling. PLoS Pathog. 2014;10:e1003954. doi: 10.1371/journal.ppat.1003954. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]; This study provides the first genetic validation of the role of a C. trachomatis T3SS effector and reveals a hierarchical order in bacterial effector translocation into host cells by differential binding to shared chaperones.
- 46. Pais SV, Milho C, Almeida F, Mota LJ. Identification of novel type III secretion chaperone–substrate complexes of Chlamydia trachomatis. PLoS ONE. 2013;8:e56292. doi: 10.1371/journal.pone.0056292. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 47. Bullock HD, Hower S, Fields KA. Domain analyses reveal that Chlamydia trachomatis CT694 protein belongs to the membrane-localized family of type III effector proteins. J. Biol. Chem. 2012;287:28078–28086. doi: 10.1074/jbc.M112.386904. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 48. Mojica SA, et al. SINC, a type III secreted protein of Chlamydia psittaci, targets the inner nuclear membrane of infected cells and uninfected neighbors. Mol. Biol. Cell. 2015;26:1918–1934. doi: 10.1091/mbc.E14-11-1530. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 49. Kokes M, Valdivia RH. In: Intracellular Pathogens 1: Chlamydiales. Tan M, Bavoil PM, editors. Vol. 1. ASM press; 2012. pp. 170–191. [ Google Scholar ]
- 50. Richards TS, Knowlton AE, Grieshaber SS. Chlamydia trachomatis homotypic inclusion fusion is promoted by host microtubule trafficking. BMC Microbiol. 2013;13:185. doi: 10.1186/1471-2180-13-185. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 51. Mital J, Lutter EI, Barger AC, Dooley CA, Hackstadt T. Chlamydia trachomatis inclusion membrane protein CT850 interacts with the dynein light chain DYNLT1 (Tctex1) Biochem. Biophys. Res. Commun. 2015;462:165–170. doi: 10.1016/j.bbrc.2015.04.116. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 52. Bocker S, et al. Chlamydia psittaci inclusion membrane protein IncB associates with host protein Snapin. Int. J. Med. Microbiol. 2014;304:542–553. doi: 10.1016/j.ijmm.2014.03.005. [ DOI ] [ PubMed ] [ Google Scholar ]
- 53. Damiani MT, Gambarte Tudela J, Capmany A. Targeting eukaryotic Rab proteins: a smart strategy for chlamydial survival and replication. Cell. Microbiol. 2014;16:1329–1338. doi: 10.1111/cmi.12325. [ DOI ] [ PubMed ] [ Google Scholar ]
- 54. Gambarte Tudela J, et al. The late endocytic Rab39a GTPase regulates multivesicular bodies–chlamydial inclusion interaction and bacterial growth. J. Cell Sci. 2015;128:3068–3081. doi: 10.1242/jcs.170092. [ DOI ] [ PubMed ] [ Google Scholar ]
- 55. Leiva N, Capmany A, Damiani MT. Rab11-family of interacting protein 2 associates with chlamydial inclusions through its Rab-binding domain and promotes bacterial multiplication. Cell. Microbiol. 2013;15:114–129. doi: 10.1111/cmi.12035. [ DOI ] [ PubMed ] [ Google Scholar ]
- 56. Schlager MA, et al. Bicaudal D family adaptor proteins control the velocity of Dynein-based movements. Cell Rep. 2014;8:1248–1256. doi: 10.1016/j.celrep.2014.07.052. [ DOI ] [ PubMed ] [ Google Scholar ]
- 57. Moorhead AM, Jung JY, Smirnov A, Kaufer S, Scidmore MA. Multiple host proteins that function in phosphatidylinositol-4-phosphate metabolism are recruited to the chlamydial inclusion. Infect. Immun. 2010;78:1990–2007. doi: 10.1128/IAI.01340-09. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 58. Moore ER, Mead DJ, Dooley CA, Sager J, Hackstadt T. The trans-Golgi SNARE syntaxin 6 is recruited to the chlamydial inclusion membrane. Microbiology. 2011;157:830–838. doi: 10.1099/mic.0.045856-0. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 59. Kabeiseman EJ, Cichos KH, Moore ER. The eukaryotic signal sequence, YGRL, targets the chlamydial inclusion. Front. Cell. Infect. Microbiol. 2014;4:129. doi: 10.3389/fcimb.2014.00129. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 60. Lucas AL, Ouellette SP, Kabeiseman EJ, Cichos KH, Rucks EA. The trans-Golgi SNARE syntaxin 10 is required for optimal development of Chlamydia trachomatis. Front. Cell. Infect. Microbiol. 2015;5:68. doi: 10.3389/fcimb.2015.00068. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 61. Kabeiseman EJ, Cichos K, Hackstadt T, Lucas A, Moore ER. Vesicle-associated membrane protein 4 and syntaxin 6 interactions at the chlamydial inclusion. Infect. Immun. 2013;81:3326–3337. doi: 10.1128/IAI.00584-13. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 62. Pokrovskaya ID, et al. Chlamydia trachomatis hijacks intra-Golgi COG complex-dependent vesicle trafficking pathway. Cell. Microbiol. 2012;14:656–668. doi: 10.1111/j.1462-5822.2012.01747.x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 63. Ronzone E, Paumet F. Two coiled-coil domains of Chlamydia trachomatis IncA affect membrane fusion events during infection. PLoS ONE. 2013;8:e69769. doi: 10.1371/journal.pone.0069769. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 64. Ronzone E, et al. An α-helical core encodes the dual functions of the chlamydial protein IncA. J. Biol. Chem. 2014;289:33469–33480. doi: 10.1074/jbc.M114.592063. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 65. Gauliard E, Ouellette SP, Rueden KJ, Ladant D. Characterization of interactions between inclusion membrane proteins from Chlamydia trachomatis. Front. Cell. Infect. Microbiol. 2015;5:13. doi: 10.3389/fcimb.2015.00013. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 66. Geisler WM, Suchland RJ, Rockey DD, Stamm WE. Epidemiology and clinical manifestations of unique Chlamydia trachomatis isolates that occupy nonfusogenic inclusions. J. Infect. Dis. 2001;184:879–884. doi: 10.1086/323340. [ DOI ] [ PubMed ] [ Google Scholar ]
- 67. Mirrashidi KM, et al. Global mapping of the Inc–human interactome reveals that retromer restricts Chlamydia infection. Cell Host Microbe. 2015;18:109–121. doi: 10.1016/j.chom.2015.06.004. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]; This study reports the first large-scale chlamydial Inc–host interactome and suggests that by sequestering retromer components Chlamydia spp. can overcome host mechanisms that typically restrict the growth of pathogens.
- 68. Aeberhard L, et al. The proteome of the isolated Chlamydia trachomatis containing vacuole reveals a complex trafficking platform enriched for retromer components. PLoS Pathog. 2015;11:e1004883. doi: 10.1371/journal.ppat.1004883. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]; This study describes the quantitative analysis of the host-cell-derived proteome of inclusions isolated from C. trachomatis and demonstrates that retromer-associated sorting nexins and other components of host cell trafficking are enriched at the inclusion.
- 69. Seaman MN. The retromer complex — endosomal protein recycling and beyond. J. Cell Sci. 2012;125:4693–4702. doi: 10.1242/jcs.103440. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 70. Fisher DJ, Fernandez RE, Maurelli AT. Chlamydia trachomatis transports NAD via the Npt1 ATP/ADP translocase. J. Bacteriol. 2013;195:3381–3386. doi: 10.1128/JB.00433-13. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 71. Gurumurthy RK, et al. Dynamin-mediated lipid acquisition is essential for Chlamydia trachomatis development. Mol. Microbiol. 2014;94:186–201. doi: 10.1111/mmi.12751. [ DOI ] [ PubMed ] [ Google Scholar ]
- 72. Reiling JH, et al. A CREB3–ARF4 signalling pathway mediates the response to Golgi stress and susceptibility to pathogens. Nat. Cell Biol. 2013;15:1473–1485. doi: 10.1038/ncb2865. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 73. Elwell CA, et al. Chlamydia trachomatis co-opts GBF1 and CERT to acquire host sphingomyelin for distinct roles during intracellular development. PLoS Pathog. 2011;7:e1002198. doi: 10.1371/journal.ppat.1002198. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 74. Derre I, Swiss R, Agaisse H. The lipid transfer protein CERT interacts with the Chlamydia inclusion protein IncD and participates to ER–Chlamydia inclusion membrane contact sites. PLoS Pathog. 2011;7:e1002092. doi: 10.1371/journal.ppat.1002092. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 75. Agaisse H, Derre I. Expression of the effector protein IncD in Chlamydia trachomatis mediates recruitment of the lipid transfer protein CERT and the endoplasmic reticulum-resident protein VAPB to the inclusion membrane. Infect. Immun. 2014;82:2037–2047. doi: 10.1128/IAI.01530-14. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 76. Cox JV, Naher N, Abdelrahman YM, Belland RJ. Host HDL biogenesis machinery is recruited to the inclusion of Chlamydia trachomatis-infected cells and regulates chlamydial growth. Cell. Microbiol. 2012;14:1497–1512. doi: 10.1111/j.1462-5822.2012.01823.x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 77. Yao J, Dodson VJ, Frank MW, Rock CO. Chlamydia trachomatis scavenges host fatty acids for phospholipid synthesis via an acyl-acyl carrier protein synthetase. J. Biol. Chem. 2015;290:22163–22173. doi: 10.1074/jbc.M115.671008. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 78. Yao J, Cherian PT, Frank MW, Rock CO. Chlamydia trachomatis relies on autonomous phospholipid synthesis for membrane biogenesis. J. Biol. Chem. 2015;290:18874–18888. doi: 10.1074/jbc.M115.657148. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 79. Yao J, et al. Type II fatty acid synthesis is essential for the replication of Chlamydia trachomatis. J. Biol. Chem. 2014;289:22365–22376. doi: 10.1074/jbc.M114.584185. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 80. Heuer D, et al. Chlamydia causes fragmentation of the Golgi compartment to ensure reproduction. Nature. 2009;457:731–735. doi: 10.1038/nature07578. [ DOI ] [ PubMed ] [ Google Scholar ]; This study reveals that C. trachomatis induces the fragmentation of the Golgi apparatus during infection to acquire lipids and replicate.
- 81. Kokes M, et al. Integrating chemical mutagenesis and whole-genome sequencing as a platform for forward and reverse genetic analysis of Chlamydia. Cell Host Microbe. 2015;17:716–725. doi: 10.1016/j.chom.2015.03.014. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]; This study describes a defined library of chemically mutagenized and sequenced chlamydial strains and its use for genetic screens.
- 82. Al-Zeer MA, et al. Chlamydia trachomatis remodels stable microtubules to coordinate Golgi stack recruitment to the chlamydial inclusion surface. Mol. Microbiol. 2014;94:1285–1297. doi: 10.1111/mmi.12829. [ DOI ] [ PubMed ] [ Google Scholar ]
- 83. Kokes M, Valdivia RH. Differential translocation of host cellular materials into the Chlamydia trachomatis inclusion lumen during chemical fixation. PLoS ONE. 2015;10:e0139153. doi: 10.1371/journal.pone.0139153. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 84. Boncompain G, et al. The intracellular bacteria Chlamydia hijack peroxisomes and utilize their enzymatic capacity to produce bacteria-specific phospholipids. PLoS ONE. 2014;9:e86196. doi: 10.1371/journal.pone.0086196. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 85. Saka HA, et al. Chlamydia trachomatis infection leads to defined alterations to the lipid droplet proteome in epithelial cells. PLoS ONE. 2015;10:e0124630. doi: 10.1371/journal.pone.0124630. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 86. Soupene E, Rothschild J, Kuypers FA, Dean D. Eukaryotic protein recruitment into the Chlamydia inclusion: implications for survival and growth. PLoS ONE. 2012;7:e36843. doi: 10.1371/journal.pone.0036843. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 87. Soupene E, Wang D, Kuypers FA. Remodeling of host phosphatidylcholine by Chlamydia acyltransferase is regulated by acyl-CoA binding protein ACBD6 associated with lipid droplets. Microbiologyopen. 2015;4:235–251. doi: 10.1002/mbo3.234. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 88. Derre I. Chlamydiae interaction with the endoplasmic reticulum: contact, function and consequences. Cell. Microbiol. 2015;17:959–966. doi: 10.1111/cmi.12455. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 89. Agaisse H, Derre I. STIM1 is a novel component of ER–Chlamydia trachomatis inclusion membrane contact sites. PLoS ONE. 2015;10:e0125671. doi: 10.1371/journal.pone.0125671. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 90. Barker JR, et al. STING-dependent recognition of cyclic di-AMP mediates type I interferon responses during Chlamydia trachomatis infection. mBio. 2013;4:e00018–13. doi: 10.1128/mBio.00018-13. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]; This study provides the first example of a Gram-negative bacterium that synthesizes its own cyclic di-AMP to activate STING and induce the production of type I interferons.
- 91. Shima K, et al. The role of endoplasmic reticulum-related BiP/GRP78 in interferon-γ-induced persistent Chlamydia pneumoniae infection. Cell. Microbiol. 2015;17:923–934. doi: 10.1111/cmi.12416. [ DOI ] [ PubMed ] [ Google Scholar ]
- 92. Mehlitz A, et al. The chlamydial organism Simkania negevensis forms ER vacuole contact sites and inhibits ER-stress. Cell. Microbiol. 2014;16:1224–1243. doi: 10.1111/cmi.12278. [ DOI ] [ PubMed ] [ Google Scholar ]
- 93. Dumoux M, Clare DK, Saibil HR, Hayward RD. Chlamydiae assemble a pathogen synapse to hijack the host endoplasmic reticulum. Traffic. 2012;13:1612–1627. doi: 10.1111/tra.12002. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 94. Volceanov L, et al. Septins arrange F-actin-containing fibers on the Chlamydia trachomatis inclusion and are required for normal release of the inclusion by extrusion. mBio. 2014;5:e01802–14. doi: 10.1128/mBio.01802-14. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 95. Patel AL, et al. Activation of epidermal growth factor receptor is required for Chlamydia trachomatis development. BMC Microbiol. 2014;14:277. doi: 10.1186/s12866-014-0277-4. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 96. Dumoux M, Menny A, Delacour D, Hayward RD. A Chlamydia effector recruits CEP170 to reprogram host microtubule organization. J. Cell Sci. 2015;128:3420–3434. doi: 10.1242/jcs.169318. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 97. Hybiske K, Stephens RS. Mechanisms of host cell exit by the intracellular bacterium Chlamydia. Proc. Natl Acad. Sci. USA. 2007;104:11430–11435. doi: 10.1073/pnas.0703218104. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]; This paper describes two distinct strategies that Chlamydia spp. use to exit the host cell at the end of the developmental cycle.
- 98. Snavely EA, et al. Reassessing the role of the secreted protease CPAF in Chlamydia trachomatis infection through genetic approaches. Pathog. Dis. 2014;71:336–351. doi: 10.1111/2049-632X.12179. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]; This study, together with reference 145 , provides genetic evidence that CPAF is dispensable for several phenotypes that were previously attributed to CPAF activity and also reveals that CPAF may have a role in dismantling the host cell prior to bacterial exit.
- 99. Yang Z, Tang L, Sun X, Chai J, Zhong G. Characterization of CPAF critical residues and secretion during Chlamydia trachomatis infection. Infect. Immun. 2015;83:2234–2241. doi: 10.1128/IAI.00275-15. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 100. Lutter EI, Barger AC, Nair V, Hackstadt T. Chlamydia trachomatis inclusion membrane protein CT228 recruits elements of the myosin phosphatase pathway to regulate release mechanisms. Cell Rep. 2013;3:1921–1931. doi: 10.1016/j.celrep.2013.04.027. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 101. Chin E, Kirker K, Zuck M, James G, Hybiske K. Actin recruitment to the Chlamydia inclusion is spatiotemporally regulated by a mechanism that requires host and bacterial factors. PLoS ONE. 2012;7:e46949. doi: 10.1371/journal.pone.0046949. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 102. Karunakaran K, Subbarayal P, Vollmuth N, Rudel T. Chlamydia-infected cells shed Gp96 to prevent chlamydial re-infection. Mol. Microbiol. 2015;98:694–711. doi: 10.1111/mmi.13151. [ DOI ] [ PubMed ] [ Google Scholar ]
- 103. Flores R, Zhong G. The Chlamydia pneumoniae inclusion membrane protein Cpn1027 interacts with host cell Wnt signaling pathway regulator cytoplasmic activation/proliferation-associated protein 2 (Caprin2) PLoS ONE. 2015;10:e0127909. doi: 10.1371/journal.pone.0127909. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 104. Sharma M, Rudel T. Apoptosis resistance in Chlamydia-infected cells: a fate worse than death? FEMS Immunol. Med. Microbiol. 2009;55:154–161. doi: 10.1111/j.1574-695X.2008.00515.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 105. Gonzalez E, et al. Chlamydia infection depends on a functional MDM2–p53 axis. Nat. Commun. 2014;5:5201. doi: 10.1038/ncomms6201. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 106. Siegl C, Prusty BK, Karunakaran K, Wischhusen J, Rudel T. Tumor suppressor p53 alters host cell metabolism to limit Chlamydia trachomatis infection. Cell Rep. 2014;9:918–929. doi: 10.1016/j.celrep.2014.10.004. [ DOI ] [ PubMed ] [ Google Scholar ]
- 107. Kun D, Xiang-Lin C, Ming Z, Qi L. Chlamydia inhibit host cell apoptosis by inducing Bag-1 via the MAPK/ERK survival pathway. Apoptosis. 2013;18:1083–1092. doi: 10.1007/s10495-013-0865-z. [ DOI ] [ PubMed ] [ Google Scholar ]
- 108. Bohme L, Albrecht M, Riede O, Rudel T. Chlamydia trachomatis-infected host cells resist dsRNA-induced apoptosis. Cell. Microbiol. 2010;12:1340–1351. doi: 10.1111/j.1462-5822.2010.01473.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 109. Chen AL, Johnson KA, Lee JK, Sutterlin C, Tan M. CPAF: a chlamydial protease in search of an authentic substrate. PLoS Pathog. 2012;8:e1002842. doi: 10.1371/journal.ppat.1002842. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]; This study reports that the proteolysis of many of the previously reported CPAF substrates occurs during the preparation of lysates from cells that are infected with Chlamydia spp. rather than in intact cells.
- 110. Bothe M, Dutow P, Pich A, Genth H, Klos A. DXD motif-dependent and -independent effects of the Chlamydia trachomatis cytotoxin CT166. Toxins (Basel) 2015;7:621–637. doi: 10.3390/toxins7020621. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 111. Brown HM, et al. Multinucleation during C. trachomatis infections is caused by the contribution of two effector pathways. PLoS ONE. 2014;9:e100763. doi: 10.1371/journal.pone.0100763. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 112. Huang J, Lesser CF, Lory S. The essential role of the CopN protein in Chlamydia pneumoniae intracellular growth. Nature. 2008;456:112–115. doi: 10.1038/nature07355. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 113. Nawrotek A, et al. Biochemical and structural insights into microtubule perturbation by CopN from Chlamydia pneumoniae. J. Biol. Chem. 2014;289:25199–25210. doi: 10.1074/jbc.M114.568436. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 114. Sun HS, Sin AT, Poirier M, Harrison RE. Chlamydia trachomatis inclusion disrupts host cell cytokinesis to enhance its growth in multinuclear cells. J. Cell Biochem. 2015;117:132–143. doi: 10.1002/jcb.25258. [ DOI ] [ PubMed ] [ Google Scholar ]
- 115. Knowlton AE, et al. Chlamydia trachomatis infection causes mitotic spindle pole defects independently from its effects on centrosome amplification. Traffic. 2011;12:854–866. doi: 10.1111/j.1600-0854.2011.01204.x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 116. Mital J, Miller NJ, Fischer ER, Hackstadt T. Specific chlamydial inclusion membrane proteins associate with active Src family kinases in microdomains that interact with the host microtubule network. Cell. Microbiol. 2010;12:1235–1249. doi: 10.1111/j.1462-5822.2010.01465.x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 117. Chumduri C, Gurumurthy RK, Zadora PK, Mi Y, Meyer TF. Chlamydia infection promotes host DNA damage and proliferation but impairs the DNA damage response. Cell Host Microbe. 2013;13:746–758. doi: 10.1016/j.chom.2013.05.010. [ DOI ] [ PubMed ] [ Google Scholar ]
- 118. Fukasawa K, Choi T, Kuriyama R, Rulong S, Vande Woude GF. Abnormal centrosome amplification in the absence of p53. Science. 1996;271:1744–1747. doi: 10.1126/science.271.5256.1744. [ DOI ] [ PubMed ] [ Google Scholar ]
- 119. Knowlton AE, Fowler LJ, Patel RK, Wallet SM, Grieshaber SS. Chlamydia induces anchorage independence in 3T3 cells and detrimental cytological defects in an infection model. PLoS ONE. 2013;8:e54022. doi: 10.1371/journal.pone.0054022. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 120. Padberg I, Janssen S, Meyer TF. Chlamydia trachomatis inhibits telomeric DNA damage signaling via transient hTERT upregulation. Int. J. Med. Microbiol. 2013;303:463–474. doi: 10.1016/j.ijmm.2013.06.001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 121. Nagarajan U. In: Intracellular Pathogens 1: Chlamydiales. Tan M, Bavoil PM, editors. Vol. 1. ASM press; 2012. pp. 217–239. [ Google Scholar ]
- 122. Darville T, O'Connell C. In: Intracellular Pathogens 1: Chlamydiales. Tan M, Bavoil PM, editors. Vol. 1. ASM press; 2012. pp. 240–264. [ Google Scholar ]
- 123. Shimada K, Crother TR, Arditi M. Innate immune responses to Chlamydia pneumoniae infection: role of TLRs, NLRs, and the inflammasome. Microbes Infect. 2012;14:1301–1307. doi: 10.1016/j.micinf.2012.08.004. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 124. Najarajan U. In: Bacterial Activation of Type I Interferons. Parker D, editor. Springer; 2014. pp. 101–102. [ Google Scholar ]
- 125. Zhang Y, et al. The DNA sensor, cyclic GMP–AMP synthase, is essential for induction of IFN-β during Chlamydia trachomatis infection. J. Immunol. 2014;193:2394–2404. doi: 10.4049/jimmunol.1302718. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]; This study demonstrates that DNA from C. trachomatis binds to cGAS to activate STING and induce type I IFN responses during infection.
- 126. Abdul-Sater AA, et al. Enhancement of reactive oxygen species production and chlamydial infection by the mitochondrial Nod-like family member NLRX1. J. Biol. Chem. 2010;285:41637–41645. doi: 10.1074/jbc.M110.137885. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 127. Itoh R, et al. Chlamydia pneumoniae harness host NLRP3 inflammasome-mediated caspase-1 activation for optimal intracellular growth in murine macrophages. Biochem. Biophys. Res. Commun. 2014;452:689–694. doi: 10.1016/j.bbrc.2014.08.128. [ DOI ] [ PubMed ] [ Google Scholar ]
- 128. Wolf K, Fields KA. Chlamydia pneumoniae impairs the innate immune response in infected epithelial cells by targeting TRAF3. J. Immunol. 2013;190:1695–1701. doi: 10.4049/jimmunol.1202443. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 129. Abu-Lubad M, Meyer TF, Al-Zeer MA. Chlamydia trachomatis inhibits inducible NO synthase in human mesenchymal stem cells by stimulating polyamine synthesis. J. Immunol. 2014;193:2941–2951. doi: 10.4049/jimmunol.1400377. [ DOI ] [ PubMed ] [ Google Scholar ]
- 130. Al-Zeer MA, Al-Younes HM, Lauster D, Abu Lubad M, Meyer TF. Autophagy restricts Chlamydia trachomatis growth in human macrophages via IFNγ-inducible guanylate binding proteins. Autophagy. 2013;9:50–62. doi: 10.4161/auto.22482. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 131. Haldar AK, et al. Ubiquitin systems mark pathogen-containing vacuoles as targets for host defense by guanylate binding proteins. Proc. Natl Acad. Sci. USA. 2015;112:E5628–E5637. doi: 10.1073/pnas.1515966112. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 132. Haldar AK, Piro AS, Pilla DM, Yamamoto M, Coers J. The E2-like conjugation enzyme Atg3 promotes binding of IRG and Gbp proteins to Chlamydia- and Toxoplasma-containing vacuoles and host resistance. PLoS ONE. 2014;9:e86684. doi: 10.1371/journal.pone.0086684. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 133. Finethy R, et al. Guanylate binding proteins enable rapid activation of canonical and noncanonical inflammasomes in Chlamydia-infected macrophages. Infect. Immun. 2015;83:4740–4749. doi: 10.1128/IAI.00856-15. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 134. Haldar AK, et al. IRG and GBP host resistance factors target aberrant, “non-self” vacuoles characterized by the missing of “self” IRGM proteins. PLoS Pathog. 2013;9:e1003414. doi: 10.1371/journal.ppat.1003414. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]; This study reveals that the intracellular immune recognition of pathogen-containing vacuoles is partly dictated by the lack of `self' IRGM proteins from these structures.
- 135. Wolf K, Plano GV, Fields KA. A protein secreted by the respiratory pathogen Chlamydia pneumoniae impairs IL-17 signalling via interaction with human Act1. Cell. Microbiol. 2009;11:769–779. doi: 10.1111/j.1462-5822.2009.01290.x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 136. Kessler M, et al. Chlamydia trachomatis disturbs epithelial tissue homeostasis in fallopian tubes via paracrine Wnt signaling. Am. J. Pathol. 2012;180:186–198. doi: 10.1016/j.ajpath.2011.09.015. [ DOI ] [ PubMed ] [ Google Scholar ]
- 137. Pennini ME, Perrinet S, Dautry-Varsat A, Subtil A. Histone methylation by NUE, a novel nuclear effector of the intracellular pathogen Chlamydia trachomatis. PLoS Pathog. 2010;6:e1000995. doi: 10.1371/journal.ppat.1000995. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 138. Olive AJ, et al. Chlamydia trachomatis-induced alterations in the host cell proteome are required for intracellular growth. Cell Host Microbe. 2014;15:113–124. doi: 10.1016/j.chom.2013.12.009. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]; This study uses a global protein stability platform (GPS) to survey changes in host protein stability in response to infection with Chlamydia spp.
- 139. Furtado AR, et al. The chlamydial OTU domain-containing protein ChlaOTU is an early type III secretion effector targeting ubiquitin and NDP52. Cell. Microbiol. 2013;15:2064–2079. doi: 10.1111/cmi.12171. [ DOI ] [ PubMed ] [ Google Scholar ]
- 140. Misaghi S, et al. Chlamydia trachomatis-derived deubiquitinating enzymes in mammalian cells during infection. Mol. Microbiol. 2006;61:142–150. doi: 10.1111/j.1365-2958.2006.05199.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 141. Claessen JH, et al. Catch-and-release probes applied to semi-intact cells reveal ubiquitin-specific protease expression in Chlamydia trachomatis infection. Chembiochem. 2013;14:343–352. doi: 10.1002/cbic.201200701. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 142. Conrad T, Yang Z, Ojcius D, Zhong G. A path forward for the chlamydial virulence factor CPAF. Microbes Infect. 2013;15:1026–1032. doi: 10.1016/j.micinf.2013.09.008. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 143. Johnson KA, Lee JK, Chen AL, Tan M, Sutterlin C. Induction and inhibition of CPAF activity during analysis of Chlamydia-infected cells. Pathog. Dis. 2015;73:1–8. doi: 10.1093/femspd/ftv007. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 144. Tan M, Sutterlin C. The Chlamydia protease CPAF: caution, precautions and function. Pathog. Dis. 2014;72:7–9. doi: 10.1111/2049-632X.12213. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 145. Dille S, et al. Golgi fragmentation and sphingomyelin transport to Chlamydia trachomatis during penicillin- induced persistence do not depend on the cytosolic presence of the chlamydial protease CPAF. PLoS ONE. 2014;9:e103220. doi: 10.1371/journal.pone.0103220. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 146. Hooppaw AJ, Fisher DJ. A coming of age story: Chlamydia in the post-genetic era. Infect. Immun. 2015;84:612–621. doi: 10.1128/IAI.01186-15. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 147. Buckner LR, et al. Innate immune mediator profiles and their regulation in a novel polarized immortalized epithelial cell model derived from human endocervix. J. Reprod. Immunol. 2011;92:8–20. doi: 10.1016/j.jri.2011.08.002. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 148. Hall JV, et al. The multifaceted role of oestrogen in enhancing Chlamydia trachomatis infection in polarized human endometrial epithelial cells. Cell. Microbiol. 2011;13:1183–1199. doi: 10.1111/j.1462-5822.2011.01608.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 149. Kintner J, Schoborg RV, Wyrick PB, Hall JV. Progesterone antagonizes the positive influence of estrogen on Chlamydia trachomatis serovar E in an Ishikawa/SHT-290 co-culture model. Pathog. Dis. 2015;73:ftv015. doi: 10.1093/femspd/ftv015. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 150. Mueller KE, Plano GV, Fields KA. New frontiers in type III secretion biology: the Chlamydia perspective. Infect. Immun. 2014;82:2–9. doi: 10.1128/IAI.00917-13. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 151. Dehoux P, Flores R, Dauga C, Zhong G, Subtil A. Multi-genome identification and characterization of Chlamydiae-specific type III secretion substrates: the Inc proteins. BMC Genomics. 2011;12:109. doi: 10.1186/1471-2164-12-109. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 152. Lutter EI, Martens C, Hackstadt T. Evolution and conservation of predicted inclusion membrane proteins in Chlamydiae. Comp. Funct. Genomics. 2012;2012:362104. doi: 10.1155/2012/362104. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 153. Mital J, Miller NJ, Dorward DW, Dooley CA, Hackstadt T. Role for chlamydial inclusion membrane proteins in inclusion membrane structure and biogenesis. PLoS ONE. 2013;8:e63426. doi: 10.1371/journal.pone.0063426. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 154. Jeffrey BM, Maurelli AT, Rockey DD. In: Intracellular Pathogens 1: Chlamydiales. Tan M, Bavoil PM, editors. Vol. 1. Washington: 2012. pp. 334–351. [ Google Scholar ]
- 155. Tam JE, Davis CH, Wyrick PB. Expression of recombinant DNA introduced into Chlamydia trachomatis by electroporation. Can. J. Microbiol. 1994;40:583–591. doi: 10.1139/m94-093. [ DOI ] [ PubMed ] [ Google Scholar ]
- 156. Binet R, Maurelli AT. Transformation and isolation of allelic exchange mutants of Chlamydia psittaci using recombinant DNA introduced by electroporation. Proc. Natl Acad. Sci. USA. 2009;106:292–297. doi: 10.1073/pnas.0806768106. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]; This study reports the first generation of stable transformants of Chlamydia spp. by allelic exchange and, together with the studies in references 157 , 163 and 166 , has helped to open the door towards easier genetic manipulation of chlamydial species.
- 157. Wang Y, et al. Development of a transformation system for Chlamydia trachomatis: restoration of glycogen biosynthesis by acquisition of a plasmid shuttle vector. PLoS Pathog. 2011;7:e1002258. doi: 10.1371/journal.ppat.1002258. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]; In this study the authors develop a new shuttle vector based on the Chlamydia cryptic plasmid and modify transformation and selection protocols to achieve stable transformants of Chlamydia spp.
- 158. Wickstrum J, Sammons LR, Restivo KN, Hefty PS. Conditional gene expression in Chlamydia trachomatis using the tet system. PLoS ONE. 2013;8:e76743. doi: 10.1371/journal.pone.0076743. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 159. Agaisse H, Derre IA. C. trachomatis cloning vector and the generation of C. trachomatis strains expressing fluorescent proteins under the control of a C. trachomatis promoter. PLoS ONE. 2013;8:e57090. doi: 10.1371/journal.pone.0057090. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 160. Bauler LD, Hackstadt T. Expression and targeting of secreted proteins from Chlamydia trachomatis. J. Bacteriol. 2014;196:1325–1334. doi: 10.1128/JB.01290-13. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 161. Weber MM, Bauler LD, Lam J, Hackstadt T. Expression and localization of predicted inclusion membrane proteins in Chlamydia trachomatis. Infect. Immun. 2015;83:4710–4718. doi: 10.1128/IAI.01075-15. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 162. Mueller KE, Fields KA. Application of β-lactamase reporter fusions as an indicator of effector protein secretion during infections with the obligate intracellular pathogen Chlamydia trachomatis. PLoS ONE. 2015;10:e0135295. doi: 10.1371/journal.pone.0135295. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 163. Nguyen BD, Valdivia RH. Virulence determinants in the obligate intracellular pathogen Chlamydia trachomatis revealed by forward genetic approaches. Proc. Natl Acad. Sci. USA. 2012;109:1263–1268. doi: 10.1073/pnas.1117884109. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]; In this study the authors use chemical mutagenesis, coupled with whole-genome sequencing and a system for DNA exchange in infected cells, to generate mutants of C. trachomatis that can be used to correlate phenotype with genotype.
- 164. Demars R, Weinfurter J, Guex E, Lin J, Potucek Y. Lateral gene transfer in vitro in the intracellular pathogen Chlamydia trachomatis. J. Bacteriol. 2007;189:991–1003. doi: 10.1128/JB.00845-06. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 165. Rajaram K, et al. Mutational analysis of the Chlamydia muridarum plasticity zone. Infect. Immun. 2015;83:2870–2881. doi: 10.1128/IAI.00106-15. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 166. Johnson CM, Fisher DJ. Site-specific, insertional inactivation of incA in Chlamydia trachomatis using a group II intron. PLoS ONE. 2013;8:e83989. doi: 10.1371/journal.pone.0083989. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]; This study reports the first application-specific intron targeting to insertionally inactivate a gene in the chromosome of C. trachomatis .
- 167. Kari L, et al. Generation of targeted Chlamydia trachomatis null mutants. Proc. Natl Acad. Sci. USA. 2011;108:7189–7193. doi: 10.1073/pnas.1102229108. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 168. Jacquier N, Viollier PH, Greub G. The role of peptidoglycan in chlamydial cell division: towards resolving the chlamydial anomaly. FEMS Microbiol. Rev. 2015;39:262–275. doi: 10.1093/femsre/fuv001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 169. Pilhofer M, et al. Discovery of chlamydial peptidoglycan reveals bacteria with murein sacculi but without FtsZ. Nat. Commun. 2013;4:2856. doi: 10.1038/ncomms3856. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 170. Liechti GW, et al. A new metabolic cell-wall labelling method reveals peptidoglycan in Chlamydia trachomatis. Nature. 2014;506:507–510. doi: 10.1038/nature12892. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]; In this study the authors use a novel click-chemistry-based approach to demonstrate for the first time that C. trachomatis has peptidoglycan and functional peptidoglycan-modifying enzymes.
- 171. Ouellette SP, et al. Analysis of MreB interactors in Chlamydia reveals a RodZ homolog but fails to detect an interaction with MraY. Front. Microbiol. 2014;5:279. doi: 10.3389/fmicb.2014.00279. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 172. Kemege KE, et al. Chlamydia trachomatis protein CT009 is a structural and functional homolog to the key morphogenesis component RodZ and interacts with division septal plane localized MreB. Mol. Microbiol. 2015;95:365–382. doi: 10.1111/mmi.12855. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 173. Jacquier N, Frandi A, Pillonel T, Viollier PH, Greub G. Cell wall precursors are required to organize the chlamydial division septum. Nat. Commun. 2014;5:3578. doi: 10.1038/ncomms4578. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 174. Packiam M, Weinrick B, Jacobs WR, Maurelli AT. Structural characterization of muropeptides from Chlamydia trachomatis peptidoglycan by mass spectrometry resolves “chlamydial anomaly”. Proc. Nat. Acad. Sci. USA. 2015;112:11660–11665. doi: 10.1073/pnas.1514026112. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]; This study provides the first structural confirmation of peptidoglycan in pathogenic chlamydiae and the evidence from this study is consistent with the study in reference 170 .
- 175. Klockner A, et al. AmiA is a penicillin target enzyme with dual activity in the intracellular pathogen Chlamydia pneumoniae. Nat. Commun. 2014;5:4201. doi: 10.1038/ncomms5201. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 176. Frandi A, Jacquier N, Theraulaz L, Greub G, Viollier PH. FtsZ-independent septal recruitment and function of cell wall remodelling enzymes in chlamydial pathogens. Nat. Commun. 2014;5:4200. doi: 10.1038/ncomms5200. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (959.5 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
- Search Menu
- Sign in through your institution
- Supplements
- Cohort Profiles
- Education Corner
- Author Guidelines
- Submission Site
- Open Access
- About the International Journal of Epidemiology
- About the International Epidemiological Association
- Editorial Team
- Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Self-Archiving Policy
- Dispatch Dates
- Contact the IEA
- Journals on Oxford Academic
- Books on Oxford Academic
Article Contents
Introduction, acknowledgements.
- < Previous
Effectiveness of chlamydia screening: systematic review
- Article contents
- Figures & tables
- Supplementary Data
Nicola Low, Nicole Bender, Linda Nartey, Aijing Shang, Judith M. Stephenson, Effectiveness of chlamydia screening: systematic review, International Journal of Epidemiology , Volume 38, Issue 2, April 2009, Pages 435–448, https://doi.org/10.1093/ije/dyn222
- Permissions Icon Permissions
Background Screening programmes are promoted to control transmission of and prevent female reproductive tract morbidity caused by genital chlamydia. The objective of this study was to examine the effectiveness of register-based and opportunistic chlamydia screening interventions.
Methods We searched seven electronic databases (Cinahl, Cochrane Controlled Trials Register, DARE, Embase, Medline, PsycINFO and SIGLE) without language restrictions from January 1990 to October 2007 and reference lists of retrieved articles to identify studies published before 1990. We included studies examining primary outcomes (pelvic inflammatory disease, ectopic pregnancy, infertility, adverse pregnancy outcomes, neonatal infection, chlamydia prevalence) and harms of chlamydia screening in men and non-pregnant and pregnant women. We extracted data in duplicate and synthesized the data narratively or used random effects meta-analysis, where appropriate.
Results We included six systematic reviews, five randomized trials, one non-randomized comparative study and one time trend study. Five reviews recommended screening of women at high risk of chlamydia. Two randomized trials found that register-based screening of women at high risk of chlamydia and of female and male high school students reduced the incidence of pelvic inflammatory disease in women at 1 year. Methodological inadequacies could have overestimated the observed benefits. One randomized trial showed that opportunistic screening in women undergoing surgical termination of pregnancy reduced post-abortal rates of pelvic inflammatory disease compared with no screening. We found no randomized trials showing a benefit of opportunistic screening in other populations, no trial examining the effects of more than one screening round and no trials examining the harms of chlamydia screening.
Conclusion There is an absence of evidence supporting opportunistic chlamydia screening in the general population younger than 25 years, the most commonly recommended approach. Equipoise remains, so high-quality randomized trials of multiple rounds of screening with biological outcome measures are still needed to determine the balance of benefits and harms of chlamydia screening.
Chlamydia screening is widely promoted in high-income countries as an intervention to prevent reproductive tract morbidity, including infertility, in women by reducing chlamydia transmission. 1–3 A National Chlamydia Screening Programme in England 1 and regional Infertility Prevention Programs in the United States 4 offer chlamydia screening to eligible, sexually active individuals younger than 25 years when they attend consultations at specified health care, or other, settings. This approach is known as opportunistic screening ( Box 1 ). The other main approach is register-based screening ( Box 1 , also known as call–recall, proactive or population based). Key features of register-based screening are an up-to-date register of those eligible for screening, which can be used to send proactive invitations for screening; identifying those who have not responded to an invitation and sending reminders; sending repeat invitations at regular defined intervals; compiling regular reports of the coverage; and follow-up of testing. 5 Register-based chlamydia screening is being piloted in three regions of the Netherlands from 2008. 6
Members of a defined population, who may not know they are at risk of a disease or its complications, are asked a question or offered a test to identify those who are more likely to be helped than harmed by further tests or treatment. 49
Screening programme
A continuing public health service that ensures screening is delivered at sufficiently regular intervals to a high enough proportion of the target population to achieve defined levels of benefit at the population level, while minimizing harm. 12
Register-based screening
Registers are used to identify and enumerate the target population (e.g. in a geographical area, practice list of a general practitioner or members of a health maintenance organization), to send invitations for screening, to send reminders to those who have not attended and to send regular repeat invitations at appropriate intervals. Invitations are sent to individuals irrespective of their record of health service use. Also known as population, proactive, call–recall, cyclical, active or systematic screening. 49
Opportunistic screening
A health professional offers a screening test to patients attending health care or other defined settings for any reason. Individuals who do not use relevant health services will not have an opportunity to be offered screening. The health professional takes responsibility for repeating the test offer at appropriate intervals. 49
The way in which screening services are organized and delivered can affect their success. Regular screening and follow-up are needed to realize sustainable population benefits. 7 This might be particularly important for communicable diseases where asymptomatic and repeated infections are common. 8 These requirements are difficult to achieve and monitor with opportunistic approaches, which require the target group to use health services regularly, practitioners to offer repeat tests at appropriate intervals and administrative systems to track individuals attending multiple screening venues. Opportunistic cervical cancer screening, offered in the 1960s and 1970s by general practitioners and family planning clinics in the United Kingdom, was ineffective. 9 Older women at highest risk were screened infrequently or not at all, whilst those at low risk were screened repeatedly. The fall in the death rate from cervical cancer, which began before screening was introduced, did not accelerate until an organized call–recall system, which increased regular coverage to 80%, was introduced in 1988. 10
The primary objective of a screening programme is to reduce mortality or morbidity. 5 , 9 The strength of evidence supporting chlamydia screening as a population-level intervention has, however, been challenged. 11 , 12 , 13 , 14 The objective of this study was to examine the research evidence about the effectiveness of screening to prevent chlamydia-associated morbidity and transmission systematically, with a focus on the organizational approach.
Data sources and searches
We searched Cinahl, Cochrane Controlled Trials Register, Database of Abstracts of Research Effectiveness, Embase, Medline, PsycINFO and SIGLE from January 1990 to October 2007. We searched the reference lists of included articles to identify additional relevant articles, including those published before 1990. We had no language restrictions. We used subject heading and free text terms that combined Chlamydia trachomatis infections or pelvic inflammatory disease with terms for screening ( Supplementary Information 1 ).
Study selection
We included studies reporting primary biological outcomes of any approach to chlamydia screening in adult women and men, and harms resulting from screening. The following were considered as primary outcomes: chlamydia incidence or prevalence; pelvic inflammatory disease, ectopic pregnancy and infertility; adverse pregnancy outcomes; neonatal morbidity or mortality; and male infertility. Psychological distress, partner violence and relationship breakdown were considered as harms.
We included systematic reviews, randomized controlled trials, non-randomized comparative studies and observational time trend studies if they included data from at least two time points before the introduction of the intervention. 15 Two reviewers screened titles and abstracts to identify potentially relevant articles. Full-text articles were then read independently. Discrepancies were resolved by discussion to reach consensus about the list of articles to include.
Data extraction and quality assessment
We used published definitions of opportunistic and register-based screening to determine the approach used in included studies ( Box 1 ). Two independent reviewers assigned the screening approach and extracted data. Discrepancies were resolved by discussion, or by consultation with a third reviewer. We used criteria published by the United Kingdom National Institute for Health and Clinical Excellence for each study design to assess the quality of reporting of the study methods. 15 Supplementary Tables 1a–d shows the details of the quality assessments and criteria.
Data synthesis and analysis
We used narrative methods to describe the evidence. If two or more trials examined the same intervention and outcome, we combined the results statistically in a meta-analysis using a random effects model. We examined statistical evidence of heterogeneity due to between-trial variation using the I 2 statistic. 16
A detailed report of this study has been published. 13
Our literature searches identified 2323 unique references ( Figure 1 ). We screened 418 full-text articles and excluded 369. Of the remaining 49 studies, we excluded 36 ( Supplementary Table 2, References s1–s36 ). 13 Seventeen of these were time trend studies that did not report data from the time period before the introduction of the screening intervention (s20–s36). Eight were controlled trials for which the outcome was screening uptake (s6, 7, 11, 13, 15–17, 19) and that have been summarized elsewhere. 13 We excluded two literature reviews that informed recommendations about screening in the United Kingdom (s1) and updated recommendations in Canada (s2) because there was no description, or reference to a description, that could determine whether or not they were systematic reviews. We included six systematic reviews, 17–22 five randomized trials, 23–27 one non-randomized comparative study 28 and one time trend study. 29
Flow diagram for results of electronic database and handsearching for articles on chlamydia screening
We found no randomized controlled trials of the effects of opportunistic chlamydia screening in non-pregnant women, pregnant women in antenatal clinics or men. We found no randomized trial reporting the outcomes of infertility in women or men, ectopic pregnancy, adverse pregnancy outcomes, neonatal morbidity or mortality and no trials that examined the effects of more than one round of any screening intervention. We found no trials reporting harms of chlamydia screening.
Systematic reviews
Four systematic reviews 17–20 directly informed published national guidelines on chlamydia screening in Canada, 17 Scotland, 18 and the United States 2 ( Table 1 ). The most recent review, by the United States Preventive Services Task Force, 20 updated an earlier full review. 19 Four reviews were based on searches of multiple electronic databases. 18 , 19 , 21 , 22 Our literature searches identified all studies cited by the reviews as evidence of effectiveness. No review separated studies according to the organizational approach to screening. Among five reviews that assessed the same trial of a register-based approach, 23 two graded this as good evidence 19 , 22 and one as fair evidence 17 to recommend screening of women at high risk of chlamydia. The Scottish guideline recommended opportunistic testing of women at high risk of chlamydia but noted that no randomized trial supported this. 18 This guideline is being updated. 30 One review graded the same study as a low-quality randomized trial with no recommendation. 21 Three reviews recommended chlamydia screening before termination of pregnancy. 18 , 21 , 22 Three reviews cited evidence from ecological time trend studies as supportive evidence in favour of chlamydia screening programmes. 19 , 21 , 22
Characteristics of systematic reviews of effectiveness of chlamydia screening on primary outcomes
GUM, genitourinary medicine; IUD, intra-uterine device; PID, pelvic inflammatory disease; RCT, randomized controlled trial; STI, sexually transmitted infection; TOP, termination of pregnancy; USPSTF, United States Preventive Services Task Force.
a Review questions are those specified by the authors. Only questions and included studies directly related to evidence for the effectiveness of screening are included here. Reviews also examined evidence for chlamydia prevalence, risk factors, accuracy of diagnostic tests and cost-effectiveness.
We found three randomized controlled trials 23–25 and one non-randomized comparative study 28 reporting the effects of register-based chlamydia screening on the incidence of pelvic inflammatory disease or on chlamydia prevalence ( Tables 2 and 3 ).
Characteristics of included studies, by screening approach, design and outcome
CCT, controlled clinical trial; DFA, direct fluorescent antibody test; EIA, enzyme immuno assay; NAAT, nucleic acid amplification test; RCT, randomized controlled trial; STI, sexually transmitted infection.
Results from included studies
CCT, controlled clinical trial; RCT, randomized controlled trial; f, female; m, male; wm, woman months; mth, months; PID, pelvic inflammatory disease.
Effects on reproductive tract morbidity in women
Two randomized controlled trials (3537 women enrolled) found that the risk of pelvic inflammatory disease in women invited to be screened was about half that of control groups 1 year after a single round of register-based screening [summary risk ratio 0.46, 95% confidence interval (CI) 0.27–0.78, I 2 = 0%]. 23 , 24 There were biases in the design of both studies ( Tables 2 and 3 , Supplementary Table 1a ). In the earliest published study, 23 the authors used the register of a health maintenance organization in the United States to identify, invite and follow-up their target population. Overall, 36 547 women were randomized first to screening and control groups, and consent for inclusion was sought if their responses to a postal questionnaire showed them to be single, non-pregnant and at high risk of chlamydia (score >3, based on age ≤24 = 1, black race = 2, nulligravid = 1, douching in past 12 months = 1 and ≥2 sexual partners in past 12 months = 1). Women randomized to the screening group only were also telephoned to increase the number with a risk assessment and allow screening appointments to be made. These practices changed the planned ratio in intervention and control groups from 1:2 to 1:1.6 (total 2607). Sixty-four per cent of women in the intervention group and an unknown proportion in the control group were screened for chlamydia. Østergaard et al. 24 conducted a cluster randomized trial in 17 high schools in Aarhus County, Denmark (8909 students). Sexually active female and male students responding to the invitation were asked to collect urine and/or vaginal specimens at home, or told that they could be tested at a local health clinic. Response rates were higher in those assigned to the intervention (32% of those randomized) than control group (24%). Participants in the intervention group were given additional information about the importance of partner notification if diagnosed with chlamydia. 31 Ascertainment of pelvic inflammatory disease was unblinded, and loss to follow-up 1 year later was nearly 50% ( Supplementary Table 1a ).
Effects on chlamydia transmission
Two randomized trials and one non-randomized comparative study reported effects of register-based screening on chlamydia prevalence, as a measure of chlamydia transmission ( Tables 2 and 3 ). 24 , 25 , 28 There were biases in all studies ( Supplementary Table 1a ), and results could not be combined statistically because of differences in the ways the data were collected and reported. Østergaard et al. 24 found fewer diagnosed infections at follow-up in female students who had been proactively invited to provide home-collected vaginal specimens compared with controls who were told that they could visit their general practitioner ( Tables 2 and 3 ). Cohen et al. 28 compared infection rates between three schools that had provided chlamydia screening over a 3-year-period with a non-randomly selected group of five schools with no screening. The infection rate in intervention compared with control schools was lower at follow-up in boys but not in girls ( Tables 2 and 3 ). In both studies, there was no baseline assessment or treatment in control groups, so, at follow-up, both incident and prevalent infections would be detected, whereas only incident infections would be detected in intervention schools. Hodgins et al. 25 invited all adults in six Inuit villages in Canada to provide urine specimens as part of an intensive sexual health education and promotion campaign. Chlamydia prevalence 1 year after screening in intervention villages fell ( Tables 2 and 3 ). In six comparison villages where there was no campaign but opportunistic testing was available, prevalence 1 year later had not changed.
We found two randomized trials (2263 women) investigating the effects of opportunistic chlamydia screening on pelvic inflammatory disease in women requesting surgical termination of pregnancy. 26 , 27 In women in Sweden offered pre-operative chlamydia screening and treatment, the risk of post-abortal pelvic inflammatory disease was about half that in women in the control group who received diagnostic testing if they had post-operative symptoms (risk ratio 0.50, 0.27–0.95, Tables 2 and 3 ). 26 Details of randomization, concealment and blinding of outcome assessment were not reported. The other study compared a strategy of pre-operative screening using an enzyme linked immunoassay followed by treatment of positive cases with universal peri-operative antibiotic prophylaxis in Scotland. 27 The study was terminated before reaching the required sample size, but there was weak evidence of more episodes of post-operative pelvic inflammatory disease at 8 weeks with the screening strategy than with universal prophylaxis (risk ratio 1.47, 0.97–2.23, Tables 2 and 3 ). Re-infection was not assessed as an outcome, but partner management in women randomized to screening was poor: of 45 women with positive chlamydia or gonorrhoea tests, 4 partners were documented to have received treatment. 27
We included one ecological study that reported time trends in diagnosed chlamydia rates in Uppsala County, Sweden. 29 Herrmann and Egger used microbiology records and population data before and after chlamydia testing in health care settings became widespread. 29 In 1988, chlamydia became a notifiable infection, and partner notification was mandatory. Five youth clinics providing free chlamydia testing and treatment were established, and there was a publicity campaign. Chlamydia testing was also available in other health care settings, but the activities were not coordinated as a screening programme. Chlamydia rates per 1000 tests were reported by year for 3 years before opportunistic testing became widely available (1985–87) and 6 years after (1988–93). The chlamydia infection rate fell in both time periods in both women and men. An increase in male rates at the end of the study period was noted as an indication of increasing incidence.
Our systematic review assessed evidence for the effectiveness of chlamydia screening in preventing chlamydia-associated morbidity or transmission of infection. Trial reporting quality was generally poor, and there were methodological weaknesses that could have biased the results of all included studies. In two randomized trials, a single round of register-based screening was associated with a reduced incidence of pelvic inflammatory disease in women at 1 year. Information about the effects of any chlamydia screening approach on transmission of infection was difficult to interpret. Trials of opportunistic chlamydia screening have only been conducted in women undergoing surgical termination of pregnancy. We found no evidence for the effectiveness of opportunistic screening in any other population, of multiple rounds of any screening approach or about the harms of chlamydia screening ( Table 4 ).
Summary of randomized controlled trial evidence of effects of chlamydia screening in non-pregnant women, pregnant women and men, on primary outcomes a
a Primary outcomes of chlamydia screening: incidence of short-term (PID) or long-term (tubal infertility or ectopic pregnancy) complications in women; adverse pregnancy outcomes; neonatal morbidity; change in chlamydia prevalence.
Strengths and weaknesses
The strengths of this review were that we conducted comprehensive literature searches of multiple databases without language restrictions, and used rigorous methods to identify, appraise and synthesize the evidence. It is, therefore, unlikely that we excluded any important studies during the dates covered by the search. The main weakness of the review was that it was not possible to combine effect estimates statistically for most comparisons because of the small numbers of studies, and differences in the interventions, populations or data reporting. Incomplete reporting of methods made it difficult to interpret the findings of many studies.
Comparison with other systematic reviews
The results of our systematic review differ from others, 17–22 which concluded that there was fair or good evidence to recommend chlamydia screening. First, by stratifying results according to the organizational approach to chlamydia screening, we showed that any evidence of a beneficial effect applied only to register-based interventions, and this was limited by poor trial quality. Second, we found some evidence that chlamydia screening in men might contribute to a reduction in the incidence of pelvic inflammatory disease in women ( Table 4 ). The cluster randomized trial by Østergaard and colleagues 31 involved both male and female students. If we presume that some students at least were in shared sexual networks, the reduction in pelvic inflammatory disease in women 24 could be attributed in part to screening and treatment of chlamydia-infected men. The United States Preventive Services Taskforce 20 reviewed the same trial but concluded that there were no studies showing that chlamydia screening in men produces benefits in women. New Canadian guidelines on sexually transmitted infections noted a gap in the evidence about chlamydia screening in men, citing the US review. 32 Third, we did not find the results of time trend studies to be consistent with randomized controlled trial results. 19 , 21 , 22 The only eligible study in our review showed that chlamydia rates were falling in Uppsala County, Sweden, before opportunistic testing became widespread. 29
Interpretation of the evidence
It has been argued that further randomized trials of the primary outcomes of chlamydia screening are unnecessary. 33 Our review suggests that clinical equipoise remains because the quality of trials so far does not allow the benefits or harms of chlamydia screening to be quantified accurately enough. 11 , 12 , 14 Both trials of register-based screening must have overestimated the effect of screening: 23 , 24 Chlamydia trachomatis is implicated in about 30% of acute pelvic inflammatory disease, 34 so even if screening and treatment could prevent all cases resulting from ascending chlamydia, a halving of the overall risk of pelvic inflammatory disease is implausible. Seven of nine cases of pelvic inflammatory disease in the trial by Scholes and colleagues 23 were in women tested for chlamydia, so the intervention did not prevent these cases. Furthermore, results from women at high risk of chlamydia in this trial might not be generalizable to all women younger than 25 years, and additional contacts with those invited for screening could have exaggerated uptake or changed behaviours, which might have increased differences in outcomes between groups. In the trial by Østergaard et al. , 24 open outcome assessment could have increased the estimated effect if symptoms were more likely to be assigned to pelvic inflammatory disease in the unscreened group and to other causes in the screened group. Differential enrolment rates and high losses to follow-up might also have resulted in systematic differences between intervention and control groups. The large effects seen in trials have not been replicated in observational studies. Rates of hospitalization for pelvic inflammatory disease among 28 000 new recruits in the United States Army were similar in screened compared with unscreened women after 18 months (relative risk, adjusted for age, race, education and aptitude 0.94, 95% CI 0.69–1.29). 35 This slight reduction and the lower overall hospitalization (adjusted relative risk 0.94, 95% CI 0.90–0.99) among screened women could reflect an unmeasured ‘healthy screenee’ effect. 5
The ways in which the interventions examined could have prevented pelvic inflammatory disease have not been examined critically. A single screening test could only have a substantial direct effect if most infections detected were recently acquired and were treated before causing upper genital tract inflammation. This is unlikely because chlamydia persists asymptomatically for up to 5 years after diagnosis, 36 so most infections in a previously unscreened population would already have been present for some time and might already have caused tubal damage. Alternatively, high enough levels of screening uptake and partner notification would interrupt community transmission and reduce exposure. Once off screening uptake of 33% among school students in half the schools in one Danish community would probably not have reduced transmission substantially, 24 and health maintenance organization members are not a geographical community, so transmission is unlikely to have been affected. 23 Neither trial reported partner treatment rates. 23 , 24 In one trial that examined pre-abortion screening, only 10% of male partners of women with either chlamydia or gonorrhoea were treated. 27 Mathematical models provide the only source of information about how chlamydia screening would prevent pelvic inflammatory disease in the long term. In these models, the reduction in pelvic inflammatory disease depends on reducing transmission at a population level by yearly repeated screening, treatment and partner notification to reduce the risk of exposure to chlamydia, and not to an individual effect of interruption of ascending infection. 37–39
Implications for chlamydia screening programmes
Distinguishing between register-based and opportunistic approaches is important for operational reasons because the way in which a screening programme is delivered in practice should reproduce the benefit to the target population observed in clinical trials. 5 In the United States, the Preventive Services Task Force requires direct evidence that the entire screening service achieves the primary health outcome. 40 In the United Kingdom 5 , 41 and New Zealand, 42 national screening committees require evidence of effectiveness from high-quality randomized trials of the screening programme that is to be delivered. In most countries that recommend chlamydia screening of specified groups of asymptomatic individuals, tests are offered opportunistically, usually in health care settings. Our review shows that published trials about opportunistic chlamydia screening provide indirect short-term evidence of inadequate quality. Even where opportunistic screening services are coordinated nationally with defined service standards, coverage of regular screening and outcomes of opportunistic screening are difficult to measure because health service data on screening uptake are not routinely linked to data on chlamydia-associated complications and neither data source is linked to population records. Current data from the best-performing region in the National Chlamydia Screening Programme in England show that, in contrast to predicted uptake of 50%, 43 only 2.5% of 16- to 24-year-olds were screened in the past year, 44 and chlamydia positivity rates remain at 10–11%. 1 There are no performance indicators for the primary outcomes. 45 The Chlamydia Screening Implementation project in the Netherlands will show whether or not the uptake of a register-based approach with repeated yearly screening invitations 6 can achieve the results observed by Scholes et al. 23 and Østergaard et al. 24
Implications for the evaluation of chlamydia screening
Uptake of screening is not an adequate surrogate endpoint for trials of chlamydia screening because the level of coverage predicting a defined reduction in morbidity or transmission is not known. 46 Objective endpoints, such as ectopic pregnancy or tubal infertility, often require invasive diagnosis and are too rare or delayed to be used realistically in trials. Pelvic inflammatory disease is the most commonly used biological outcome because it is the most frequent acute complication of lower genital tract chlamydia and is strongly associated with impaired fertility. 34 Clinical diagnosis is, however, known to be insensitive, non-specific and subjective. 34 , 47 If misclassification applies similarly to both screened and unscreened groups, the effect size would be attenuated. The diagnosis of lower abdominal symptoms could, however, be different in screened and unscreened women if the investigator is influenced by the chlamydia screening status. Since practitioners usually cannot be blinded to the screening allocation in trials, symptoms reported in follow-up consultations should be recorded in a standard way, with the final outcome assessment made by an independent blinded committee.
A reduction in chlamydia transmission, attributable to screening, would provide good primary evidence of effectiveness. Comparing chlamydia test positivity after a single screening round biases the result in favour of the screened group, which includes incident infections, while infections in the control group include prevalent infections that might have been present before the trial started. Ideally, the effect of chlamydia screening on chlamydia transmission would be determined in a population in whom prevalent infections had been detected and treated, for example following a prevalence study with high participation, follow-up, treatment and partner notification rates. The chlamydia screening intervention would then be implemented in randomly assigned areas over two or more screening intervals. The final comparison would be made between screened and unscreened communities in a follow-up prevalence survey.
This systematic review provides information about the limitations of published evidence about the effectiveness of chlamydia screening, which can be used to inform future research and decisions about the introduction of chlamydia screening programmes. Where chlamydia screening interventions have already been introduced, our findings can be used to help design studies to determine the most effective way to deliver and monitor the outcomes of chlamydia screening. Interventions that combine the advantages of both register-based and opportunistic screening approaches could reach a higher proportion of the target population than either method alone. 48 For example, regular postal invitations could be supplemented with opportunistic offers to eligible individuals who have not responded. Alternatively, an initial opportunistic offer of testing could be followed up by postal invitations to non-attenders. The effectiveness and cost-effectiveness of chlamydia screening require further evaluation in randomized trials over multiple screening rounds with primary biological endpoints to show that the programme does more good than harm at reasonable cost.
United Kingdom National Institute for Health and Clinical Excellence.
N.L., N.B., L.N. and A.S. are, or have been, employed by the University of Bern, which received funding from the United Kingdom National Institute for Health and Clinical Excellence (NICE). Parts of the research referred to in this article were commissioned by NICE to inform the development of its guidance on the prevention of sexually transmitted infections and under-18 conceptions. The opinions expressed in the article are those of the authors and not the institute. This article does not constitute NICE guidance. The authors thank Shelagh Redmond for assistance with bibliographic and database management for the review.
Conflict of interest: None declared.
Chlamydia screening is widely believed to be an effective and cost-effective intervention to improve reproductive and sexual health.
The results of randomized controlled trials have overestimated the benefits of chlamydia screening on preventing pelvic inflammatory disease.
The chlamydia screening interventions that have been evaluated in randomized controlled trials are not those that are implemented in practice.
Clinical equipoise about the balance of benefits and harms of chlamydia screening programmes remains.
Google Scholar
Google Preview
Supplementary data
Email alerts, citing articles via, looking for your next opportunity.
- About International Journal of Epidemiology
- X (formerly Twitter)
- Recommend to your Library
Affiliations
- Online ISSN 1464-3685
- Copyright © 2024 International Epidemiological Association
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.

IMAGES
COMMENTS
Chlamydia is a sexually transmitted infectious disease caused by the bacterium Chlamydia trachomatis. In the United States, it is the most commonly reported bacterial infection. Globally, it is the most common sexually transmitted infection. It causes an ocular infection called "trachoma," which is the leading infectious cause of blindness worldwide.
Chlamydia is a sexually transmitted infectious disease caused by the bacterium Chlamydia trachomatis. In the United States, it is the most commonly reported bacterial infection. Globally, it is the most common sexually transmitted infection. It causes an ocular infection called "trachoma," which is the leading infectious cause of blindness ...
Chlamydia trachomatis infection is the most frequently reported bacterial sexually transmitted infection (STI) globally, with an estimated 131 million new cases occurring annually. Chlamydia spreads through vaginal, anal, or oral sex with a partner with the infection. It is common among young people aged 15-24 years. However, as chlamydia infections are often asymptomatic, most infected are ...
1.1. Design. We used a pre/post‐test design and aimed to increase chlamydia knowledge among undergraduate students through web‐based education in the fall 2018 semester. The setting was a mid‐size, private, co‐educational, U.S. university. A convenience sample of students was recruited via flyers and emails.
INTRODUCTION. Chlamydia trachomatis (CT) ... Yvonne Sweeney and other members of the University of Washington Chlamydia Research Group for their enlightening conversations that formed the basis of this review paper. FUNDING. This work was supported by the National Institutes of Health (NIH) [grant R21AI142369 to C.M.K. and L.A.B.] and the King ...
INTRODUCTION. Chlamydia trachomatis is the most common bacterial sexually transmitted infection and causes significant morbidity and economic burden globally ().There are approximately 131 million new cases of chlamydial infection occurring annually in individuals aged 15-49 years, with an incidence rate of 38 per 1,000 women and 33 per 1,000 men.
Chlamydia trachomatis and Chlamydia pneumoniae, the major species that infect humans, are responsible for a wide range of diseases 2,4 and will be the focus of this Review. Strains of C. trachomatis are divided into three biovars and are further subtyped by serovar. The trachoma biovar (serovars A-C) is the leading cause of non-congenital ...
Introduction. Chlamydia screening is widely promoted in high-income countries as an intervention to prevent reproductive tract morbidity, including infertility, in women by reducing chlamydia transmission. 1-3 A National Chlamydia Screening Programme in England 1 and regional Infertility Prevention Programs in the United States 4 offer chlamydia screening to eligible, sexually active ...
A prospective cohort study evaluating women with rectal chlamydia found improved cure rates with doxycycline (95.5%) compared with azithromycin (78.5) (p < 0.001). 87 In a retrospective chart review of 526 men and women with rectal chlamydia, among those who presented for re-testing, the reinfection rate was 5.8% in those treated with ...
Chlamydia infects about 131 million people worldwide each year and is the second leading cause of more than 1 million new sexually transmitted infections (STIs) that occur daily; globally, around 357 million new STIs are reported each year (World Health Organization [WHO], 2019). In the United States (U.S.), the Centers for Disease Control and ...