

ALLIANCE FOR HUMAN RESEARCH PROTECTION
Advancing Voluntary, Informed Consent to Medical Intervention

- Board of Directors
- Distinguished Advisory Board
- Honor Roll–Exemplary Professionals
- First, do no Harm
- Human Rights
- Informed Consent
- Nuremberg Code
- Discrimination
- Medicalized Racism
- Gene Modification
- Depopulation
- Technocracy
- Propaganda – Censorship
- Clinical Trials
- Concealed Data
- Public-Private Partnerships
- Pharma Corrupt Influence
- Publication Bias
- Organ Harvesting
- Bioweapon Experiments
- Transhumanism
- Current Medical Atrocities
- Japanese Atrocities
- Nazi Atrocities
- Operation Paperclip
- CIA Mind-Control
- CIA Torture
- U.S. Radiation Experiments
- Unethical Experiments
- Pandemic Control
- Great Reset
- Diabolical Lockstep
- Apartheid Policies
- Coronavirus Fear
- Covid Pandemic
- Government Overreach
- Vaccine Profit Engine
- Child Sacrifice
- Vaccine Mandates
- Vaccine Risks
- Vaccine Safety
Children were the raw material of medical research /Newborn Screening for 29 conditions
Miscellaneous
Children were the raw material of medical research – CBS 60 Minutes /Newborn Screening for 29 conditions – NYT
Mon, 28 Feb 2005
Children have historically been the voiceless victims of medical research abuse – and the doctors and staff who abused them have almost never been held accountable – they are shielded by a whitewashed wall of silence. On Feb. 9, CBS 60 Minutes reported about the buried secrets at Sonoma State Hospital (now Sonoma Developmental Center), where 3,500 children with disabilities lived in the 1950s and 1960s. The children were used in medical experiments without parental informed consent – they were subjected to government-sponsored radiation experiments, among others. Susan Lederer, who teaches medical history at Yale University, and was a member of President Clinton’s Advisory Commission on Human Radiation Experiments, told 60 Minutes that the researchers and staff regarded the children as “the raw material of medical research.” When they died researchers acquired their brains, also without consent.
Despite the institution’s continued denial that such experiments took place, the facts were uncovered by Karen Alves who spent 12 years on a hunt to find out what happened to her little brother, Mark, who had cerebral palsy and was sent to Sonoma in 1958, at age 3. He died in 1961, when he was 6 years old – no death certificate had been issued. Mark was one of 1,100 Sonoma State cerebral palsy patients who were experimented on from 1955-1960. Karen Alves wasn’t able to find out what tests Mark was subjected to. But she found a document that showed that her brother had been part of the study, assigned Specimen #8732. Karen discovered that patients in the study were put through painful procedures like the pneumoencelphalogram, in which air is injected into the brain before a series of X-rays. “Imagine puncturing someone’s spinal cord, drawing fluid out and putting a foreign substance in there. Gas,” says Karen. “When they trap air in your body, you’re in pain, excruciating pain, for days.”
After a two year battle to obtain her brother’s medical records, a court order finally forced Sonoma to release them. Although incomplete, Karen found that her brother had suffered horribly before he died– most likely as a result of the radiation experiment:
The record indicated he had suffered from unusually high fevers the last six months of his life before dying of a seizure. Karen notes that “Swollen eyes, seizures, those things can fit in with radiation poisoning.” She also discovered that “They took my brother’s brain without consent, and the doctor, in his obituary it said that he had one of the largest brain collections,” says Karen.
Lederer told 60 Minutes that she wasn’t shocked by the findings because "researchers have been using disabled children in experiments for over a century." She acknowledges that the experiments were not intended, nor were they, of any benefit to the children who served as mere guinea pigs. Lederer said that using captive populations meant big money for medical researchers: “It would even be an advantage in applying for grant money, because you don’t have to go to the problem of recruiting subjects.” In the case of Sonoma State, records show that when the study began, cerebral palsy admissions there jumped by 300 percent.
Even today, the medical research establishment and those who set government health care policy appear to have learned little from the lessons of the radiation experiments. A report in The New York Times (Feb 21) reveals that "An influential federal advisory group plans to recommend in the next few weeks that all newborns be screened for 29 rare medical conditions." Some of the conditions are well known, like sickle cell anemia, some obscure, affecting less than 100 infants a year. Those who want to screen the infants offer no known treatment for all but 5 of the conditions to be screened, and no medically justifiable rationale for screening.
Responsible medical experts oppose such screening – the challenge is to ensure that the commercial interests of screening proponents do not prevail. Dr. Norman Fost, a professor of pediatrics and director of the program in medical ethics at the University of Wisconsin, points out: “The majority of newborn screening tests have failed. Over the years, thousands of normal kids have been killed or gotten brain damage by screening tests and treatments that turned out to be ineffective and very dangerous.” He recounts the harmful consequences from premature screening for PKU, an enzyme deficiency which, in affected infants, can cause brain damage. But screening for PKU in the 1960s did not distinguish between true PKU and benign versions for whom treatment caused harm. Screening resulted in healthy babies being harmed from a prescribed low phenylalanine diet, causing them a deficiency of this essential amino acid. The American Academy of Pediatrics wrote to the secretary of health, education and welfare stating: “There is a big problem here. We don’t know what a true positive test means. We can’t distinguish a true positive from a false positive, and we don’t know what the right dose of the diet is. Mandatory screening programs should be stopped.’ ”
Given the lack of knowledge about these conditions, the inaccuracy of most screening tests, and the lack of proven treatments for most of these conditions, the risk / benefit ratio is negative, putting babies at unjustifiable risk. Dr. Lainie Friedman Ross, a pediatrician and medical ethicist at the University of Chicago, said: “We don’t know if they are medical conditions. We don’t know what to do with the information." Most conditions for which a baby may carry a genetic marker will never actually develop. Both sides agree that the tests "unintentionally pick up about 25 other conditions, in addition to the 29 that the screening is intended to find. These additional conditions show up as abnormalities, but no one knows what they mean. It is not known whether they are associated with a disease or, if so, what the effects will be."
Yet, despite the absence of a medical justification for mass screening, "It’s going like a house on fire.” Indiscriminate screening is an ill-advised irresponsible policy. Acceding to researchers’ demand for access to the DNA of newborns exposes infants to unnecessary, even harmful treatments – babies who would otherwise have led normal lives may become prisoners of medical providers. The Times reports that "in most states today, parents are not asked if they want their babies tested, though they have the right to decline it; it is simply done, with the cost, about $70 to $120, built into their hospital bills."
Another ill-advised, government sponsored screening initiative was recommended by the President’s New Freedom Commission on Mental Health– the entire population is to be screened for undetected mental health disorders – even though no valid, objectively verifiable screening tools exist. Check the AHRP website for information. We will provide updates on efforts to stop the madness of unproven medical tests and interventions
60 Minutes: A Dark Chapter In Medical History “They were the raw material of medical research.” Feb. 9, 2005
Karen Alves was just 10 when she lost her baby brother, Mark, in 1961. Mark, who suffered from cerebral palsy, was sent to Sonoma State Hospital. (Photo: CBS) As the oldest of four, she says her fondest childhood memories are of doting on her little brother. “One of the things we looked forward to, when we came home from school, was to play with Mark,” she says. But life would be a struggle for the Dal Molins because Mark was born with cerebral palsy, a condition that cripples the body, but not necessarily the mind. “In the ’50s, cerebral palsied children were considered to be developmentally disabled, mentally retarded,” says Alves to correspondent Vicki Mabrey.
“I never believed he was mentally retarded. When you looked into his eyes, he communicated through his eyes. Š He’d laugh and giggle and kick, and just screech when he saw us.” But by 3, Mark could neither walk nor talk, which meant his mother, Rosemarie, had to care for him. “We know he recognized everybody,” says Rosemarie. “He would laugh or he would cry if he was unhappy.”
The children’s father, Bill Dal Molin, felt that Rosemarie was neglecting their three daughters, because of Mark. “His mother was very, very much attentive to him, and the girls, I felt, were like troops to her,” says Bill. “She was very hard on them, the girls.”
Doctors advised the Dal Molins to commit their son, so Bill told Rosemarie they had to send Mark to an institution. It was November 1958. “I just remember one day coming home from school and the house was very quiet,” says Karen, who never got to say goodbye to her brother. “I don’t remember much after that. It profoundly affected me.”
Rosemarie had committed 3-year-old Mark to Sonoma State Hospital, the largest institution for children in California. At the time, the hospital housed 3,500 children with diverse needs, from babies born with minor defects, like a cleft palate or a club foot, to children with epilepsy and Down syndrome. While the severely disabled languished in overcrowded rooms, the able-bodied were put to work in the institution’s dairies and orchards. Rosemarie did something more that other parents who had committed their children to Sonoma State did not; she visited her son every Wednesday. “It was just a small thing that I can still do is to go see him,” says Rosemarie. “Because most of these children, they never see parents again.”
But those visits came to an abrupt end on Memorial Day, 1961, when Mark was 6. “I picked up the phone and I heard a voice say, ‘Is Mrs. Dal Molin in?’ and I just knew,” says Karen. “They didn’t even say where they were calling from. But I just, this dread came into my heart, and I got my mom and I left. I ran. I hid. Nobody told me. I knew he was dead.” From that day on, Karen and her sisters, Chris and Gail, say they never spoke Mark’s name again. They buried their grief, grew up and had families of their own. But after 40 years, they still struggle with the decision to institutionalize their brother. “It pretty much blew the family apart,” says Gail. “I believe that Dad did what he felt was best for the family. In my heart, I know that is true. But the impact of it on each one of us and the family was devastating.”
In 1994, haunted by thoughts of her baby brother, Karen decided to devote all her spare time to answering the question that had burdened her for decades: how exactly did Mark die? “I just needed to know and, no matter what it was, I needed to know. So I went to the recorder’s office,” says Karen. “There was no death certificate. One of the clerks came over to the front desk, leaned over and said ‘When did he die?’ And I said, ‘1961.’ ‘Well, when did he go into Sonoma State?’ And I said, ‘1958,’ and she said, ‘You better look into it, because strange things happened there.'”
Things got stranger still when Karen noticed an article in the local paper saying 16,000 people, including children, had been used in radiation experiments. “Out of curiosity, I started to read it, and they mentioned patients that were in state-run hospitals being used,” says Karen. “And I just go, ‘Oh my God.’ This could be it.”
Then, President Clinton had just ordered thousands of secret documents on government-sponsored human radiation experiments declassified and made available on the Internet. Karen found a study funded by the federal government involving 1,100 Sonoma State cerebral palsy patients from 1955-1960. One document she also found showed that her brother had been part of the study, assigned Specimen #8732.
Karen wasn’t able to find out what tests, if any, Mark was subjected to. But some of the patients in the Sonoma State study were put through painful procedures like the pneumoencelphalogram, in which air is injected into the brain before a series of X-rays. “Imagine puncturing someone’s spinal cord, drawing fluid out and putting a foreign substance in there. Gas,” says Karen. “When they trap air in your body, you’re in pain, excruciating pain, for days.”
“They were the raw material of medical research,” says Susan Lederer, who teaches medical history at Yale University. She was a member of the presidential committee that investigated the radiation experiments, and she says she wasn’t shocked by the findings because researchers have been using disabled children in experiments for over a century. “Children in orphanages, children in homes of the mentally retarded, these are all good populations from the sense of medical research, because you have an easily accessible group of people living in controlled circumstances, and you can monitor them,” says Lederer.
Lederer read the study that was conducted at Sonoma State Hospital, and says the children underwent painful experimentation “for which they received no direct benefit.” “It seems clear that these were intended to enlarge knowledge about cerebral palsy,” adds Lederer. It did not produce a breakthrough, although Lederer says studies using mentally retarded children were critical in creating vaccines for polio and hepatitis.
Lederer says using captive populations meant big money for medical researchers: “It would even be an advantage in applying for grant money, because you don’t have to go to the problem of recruiting subjects.” In the case of Sonoma State, records show that when the study began, cerebral palsy admissions there jumped by 300 percent. “One of the ways that medical directors of such institutions sort of connected themselves to the world of medical research was simply to provide their patients as commodities,” says Lederer. “I mean, we can provide this many guinea pigs for you.”
Sonoma State is now known as Sonoma Developmental Center. During her 12-year search, Karen repeatedly wrote to the current administrator, looking for information about Mark. She was told that there were “no records on radiation studies at Sonoma,” and that there was “no record that your brother was involved in radiation research.” “And I’d say, ‘Just go to the human radiation Web site and put in Sonoma State Hospital in your search and documents come up,” says Karen. “You’ve gotta have something there. No. They deny it. Deny it. If I called her right now, she’d deny it.”
Administrator Theresa Murphy has worked at Sonoma State for 30 years. She said she didn’t have any information about the medical experimentation that was taking place at the institution. When asked if patients at state hospitals were used in medical research, Murphy says, “I’ve read that there has been things like using rattlesnake venom of epilepsy. But you know, there’s just nothing in our archives about the research you are talking about.” “If these studies were being done, if there are patients from here being sent for radiation studies, is that a stain on the hospital record,” asks Mabrey. “I think in the history of people with developmental disabilities, and there have been some dark times. I truly believe that,” says Murphy. “And it wouldn’t surprise me that there were things we would find – consider questionable today.”
It took two years and a court order for Karen to get Sonoma State to turn over Mark’s medical records. Though not complete, records did show that Mark Dal Molin suffered unusually high fevers the last six months of his life before dying of a seizure. “He ran extremely high fevers that none of us here right now would live through,” says Karen. “Swollen eyes, seizures, those things can fit in with radiation poisoning.” Mark’s records contained another shock. Karen found not one, but two autopsy reports, one for his body and another for his brain. Karen says that Mark’s brain was removed after he died. “They took my brother’s brain without consent, and the doctor, in his obituary it said that he had one of the largest brain collections,” says Karen. “And if there’s any way for me to find that, I would like to put him back together.”
60 Minutes Wednesday learned that between 1955 and 1960, the brain of every cerebral palsy child who died at Sonoma State was removed and studied.
Rosemarie says she never gave them permission to take Mark’s brain for research purposes. “I came from Europe after the war, where all these horrendous things happened,” says Rosemarie. “I never dreamed that in this country, they would do experimenting children. Handicapped children.” Unless their families claimed them, the children ended up in a community grave with the ashes of 500 other people, or buried in a empty field without a headstone to mark their passing.
Theresa Murphy showed 60 Minutes Wednesday the final resting place of 1,400 Sonoma State patients. “The folks that remain here are undisturbed and available for family visitation,” says Murphy. But Mark Dal Molin’s family was able, at least, to spare him that fate. They had him cremated and placed his ashes in a private mausoleum.
© MMV, CBS Worldwide Inc. All Rights Reserved.
THE NEW YORK TIMES February 21, 2005 Panel to Advise Testing Babies for 29 Diseases By GINA KOLATA
An influential federal advisory group plans to recommend in the next few weeks that all newborns be screened for 29 rare medical conditions, from the well known, like sickle cell anemia, to diseases so obscure that they are known to just a handful of medical specialists and a few dozen devastated families.
But while no one argues with the idea of saving babies, the proposed screening is generating fierce debate. The dispute centers on how useful the test findings would be. Would going ahead with the full list of tests result in more good than harm, physically and emotionally? Or would it be better to forgo most of them?
Proponents say that the diseases are terrible and that an early diagnosis can be lifesaving. When testing is not done, parents often end up in a medical odyssey to find out what is wrong with their child. By the time the answer is in, it may be too late for treatment to do much good. But opponents say that for all but about five or six of the conditions, it is not known whether the treatments help or how often a baby will test positive but never show signs of serious disease. There is a danger, they say, of children with mild versions of illnesses being treated needlessly and aggressively for more serious forms and suffering dire health consequences. And both sides agree that the tests unintentionally pick up about 25 other conditions, in addition to the 29 that the screening is intended to find. These additional conditions show up as abnormalities, but no one knows what they mean. It is not known whether they are associated with a disease or, if so, what the effects will be.
The federal advisory group recommended informing the parents of such results. But that advice, too, is controversial. “Giving parents the result, saying, ‘Here’s the mutation; we are not sure what the outcome will be,’ is better than not telling,” said Sharon Terry, president and chief executive of the Genetic Alliance, an advocacy group for people with genetic disorders. Ms. Terry said it was paternalistic for doctors to presume that it was better for parents not to know.
Dr. R. Rodney Howell, a professor of pediatrics at the Leonard M. Miller School of Medicine at the University of Miami and the chairman of both the committee that wrote the report and the federal advisory group, agreed. “Do I feel it will be difficult for physicians and caretakers to deal with this?” Dr. Howell said. “The answer is yes. But I just don’t think it is proper for us to have information about an abnormality without conveying it.” But Dr. Lainie Friedman Ross, a pediatrician and medical ethicist at the University of Chicago, said: “We don’t know if they are medical conditions. We don’t know what to do with the information. Reporting test data for which there are no systems in place for follow-up testing and treatment is not rejecting paternalism, but it is patient abandonment.” In any event, Dr. Howell said, noting that states were plunging into testing programs: “It’s not really a question of, ‘Should we expand newborn screening?’ It’s happening. It’s going like a house on fire.”
In most states today, parents are not asked if they want their babies tested, though they have the right to decline it; it is simply done, with the cost, about $70 to $120, built into their hospital bills. Dr. Howell said the idea of the new recommendations was “to try to organize the programs and to try to be consistent from state to state.” “Some states screen for four conditions; others screen for 35,” said Dr. Michael S. Watson, the federal project’s director and the executive director of the American College of Medical Genetics. “A family can have their first child in one state where 25 conditions are screened and then move to another where only four are screened.”
Yet, critics say, the fact that testing is happening does not mean that it should be expanded. The history of newborn screening, they say, is filled with cautionary tales.”The majority of newborn screening tests have failed,” said Dr. Norman Fost, a professor of pediatrics and director of the program in medical ethics at the University of Wisconsin. Over the years, Dr. Fost said, “thousands of normal kids have been killed or gotten brain damage by screening tests and treatments that turned out to be ineffective and very dangerous.” To those who ask what is wrong with simply doing every available screening test, Dr. Fost tells what happened with PKU, the first genetic screening test for newborns. Today every state tests for PKU, or phenylketonuria, and it is widely acknowledged as the perfect example of screening that saves lives and prevents disability. But Dr. Fost says that a few decades ago, the situation was not nearly so rosy.
FAIR USE NOTICE: This may contain copyrighted (© ) material the use of which has not always been specifically authorized by the copyright owner. Such material is made available for educational purposes, to advance understanding of human rights, democracy, scientific, moral, ethical, and social justice issues, etc. It is believed that this constitutes a ‘fair use’ of any such copyrighted material as provided for in Title 17 U.S.C. section 107 of the US Copyright Law. This material is distributed without profit.
Subscribe To Our Newsletter!
Sign up and be the first to find out the latest news and articles about what's going on in the medical field.
You may also like
October 18, 2024
To Obey or Not Obey with Holocaust Survivor Vera Sharav
Vera Sharav is a Holocaust survivor and founder of the Alliance for Human Research Protection. She’s spent
Palestine – A Globalist Genocide – WAGE PEACE with Shabnam Palesa Mohamed and Vera Sharav (Part 1/3)
PART 1: “Why are you anti-war? Because war is anti-human. Palestine is a globalist genocide.” Vera Sharav.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Special Article
- Published: 01 October 2005
The Evolution of Neonatology
- Alistair G S Philip 1
Pediatric Research volume 58 , pages 799–815 ( 2005 ) Cite this article
24k Accesses
137 Citations
18 Altmetric
Metrics details
In 1960, the terms “neonatology” and “neonatologist” were introduced. Thereafter, an increasing number of pediatricians devoted themselves to full-time neonatology. In 1975, the first examination of the Sub-Board of Neonatal-Perinatal Medicine of the American Board of Pediatrics and the first meeting of the Perinatal Section of the American Academy of Pediatrics were held. One of the most important factors that improved the care of the neonate was the miniaturization of blood samples needed to determine blood gases, serum electrolytes, glucose, calcium, bilirubin, and other biochemical measurements. Another factor was the ability to provide nutrition intravenously, and the third was the maintenance of normal body temperature. The management of respiratory distress syndrome improved with i.v. glucose and correction of metabolic acidosis, followed by assisted ventilation, continuous positive airway pressure, antenatal corticosteroid administration, and the introduction of exogenous surfactant. Pharmacologic manipulation of the ductus arteriosus, support of blood pressure, echocardiography, and changes in the management of persistent pulmonary hypertension, including the use of nitric oxide and extracorporeal membrane oxygenation, all have influenced the cardiopulmonary management of the neonate. Regionalization of neonatal care; changes in parent–infant interaction; and technological changes such as phototherapy, oxygen saturation monitors, and brain imaging techniques are among the important advances reviewed in this report. Most remarkable, a 1-kg infant who was born in 1960 had a mortality risk of 95% but had a 95% probability of survival by 2000. However, errors in neonatology are acknowledged, and potential directions for the future are explored.
Similar content being viewed by others
Care of the critically ill neonate with hypoxemic respiratory failure and acute pulmonary hypertension: framework for practice based on consensus opinion of neonatal hemodynamics working group
Hemodynamic consequences of respiratory interventions in preterm infants
Pneumothorax in a term newborn
“There is need for specialization in neonatal medicine. This applies to doctors and nurses as well as teaching and construction of hospitals. The specialist in neonatal diseases and the nurse intensively trained and expert in the management of delicate newborns will be commonplace ere long.”
J.W. Ballantyne, 1923 ( 1 )
“In previous times the problems of the newborn child have been the province of the obstetrician, a field in which he has taken comparatively little interest and to which he has contributed little. As pediatricians we have but scratched the surface.”
C.G. Grulee, 1939 ( 2 )
The beginning of modern neonatology started ∼ 50 y ago, although there were physicians who were interested in the welfare of the newborn infant earlier. The first edition of the booklet entitled “Standards and Recommendations for Hospital Care of Newborn Infants” was published by the Committee on Fetus and Newborn of the American Academy of Pediatrics (AAP) in 1948. Four years later, Virginia Apgar presented a paper to the anesthesia research societies about neonatal assessment in the delivery room ( 3 ) and helped focus attention on the newborn infant.
Much of what we know about the years before this has been recorded in two books written by pediatricians. The first was by Thomas E. Cone, Jr., of Boston, who published his book in 1985 ( 4 ). A more concise version of Dr. Cone's thoughts can be found in the first of two small volumes published in 1983, which were widely distributed at the time but difficult to find now ( 5 ). The second was by Murdina M. Desmond, published in 1998 ( 6 ), the title of which reflects her own background.
Physicians who had an interest in the newborn were evident in the 19th century, but most were obstetricians. The idea that premature infants could be treated was probably introduced in the second half of the 19th century. At that time, considerable attention was devoted to accurate measurement of birth weight, to feeding, and to the use of incubators in France, Germany, and England.
In the mid-20th century, many pediatricians demonstrated an interest in the newborn; the primary responsibility for these infants passed from the obstetrician to the pediatrician. Several premature nurseries existed in the United States in the 1930s and 1940s, and books were written on care of the newborn, particularly the premature infant, such as those by Julius H. Hess, M.D. ( 7 ), Arthur H. Parmelee, Sr. ( 8 ), and Ethel C. Dunham ( 9 ) in the United States and V. Mary Crosse ( 10 ) in the United Kingdom. In Scandinavia, Arvo Ylppö, the Finnish pediatrician (1887–1992), spread the word about the physiology and pathology of the newborn, based on his studies in Berlin.
The work of Sir Joseph Barcroft in the United Kingdom brought new understanding of the fetus ( 11 ); and in the United States, Clement Smith published his book on the physiology of the newborn infant ( 12 ). Their work and that of their collaborators provided a solid basis for the development of evidence-based clinical care, even if the evidence was largely in nonhuman mammalian fetuses and neonates. It is appropriate to single out Dr. Julius Comroe at the Cardiovascular Research Institute in San Francisco and Dr. Geoffrey Dawes in Oxford, England ( 13 ), as key figures who fostered and promoted physiologic investigation of the fetus and neonate.
The term neonatology was coined in 1960 and is attributed to Alexander Schaffer, M.D., who used the term in the introduction to the first edition of his book ( 14 ). Also in the early 1960s, an important distinction was made between small infants who were born preterm (<38 wk gestation) and term infants who were small because of intrauterine growth restriction (IUGR). Previously, any infant whose birth weight was <2500 g was deemed to be premature.
Theoretical knowledge was advancing rapidly in this era, but it was not until the late 1960s and early 1970s that major changes in clinical care occurred. One of the most important advances was the miniaturizing of blood samples needed to do blood tests for management of clinical care, such as serum electrolytes, blood gases, bilirubin, and liver function tests ( 15 ). It was not until the second half of the 20th century that attitudes began to change. As noted by Nicholas Nelson, M.D., in 1983, “The fluid and electrolyte supports introduced by Usher and the respiratory supports introduced by Stahlman, Swyer, Tooley, James and others, not to mention the freer use of diagnostic catheters by cardiologists, all brought the sick premature newborn infant to his present state of respectability as a patient to be cared for, rather than an object to be pitied” ( 16 ).
There are several key areas that provide the basis for the care of all neonates. I have put these into perspective and focus on some of the more important advances of the last 40 y or so that pertain to the preterm infant.
THERMOREGULATION
At this point, it is probably not necessary to detail the development of the modern incubator ( 17 – 19 ). Suffice it to say that at the end of the 19th century, infant and neonatal mortality was alarmingly high and temperature regulation improved survival. Tarnier and Budin, in Paris, emphasized the utility of the incubator, as did von Reuss, in Germany, but they acknowledged the work of others who preceded them. The first incubator was introduced in 1835 by Von Ruehl in St. Petersburg, Russia ( 20 ). In the United States, attention was drawn to the potential of incubators by the side-show displays of Martin Couney at a permanent exhibit at Coney Island, which closed in 1933, and at World's Fairs in Chicago and New York (the latter in 1939–1940) ( 17 , 18 ). Various incubators were designed and used in Europe and the United States, but it was not until the work of William Silverman, Richard Day, and colleagues at Columbia, New York, in the 1950s that the benefits of modifying body temperature were demonstrated. In one of the first randomized, controlled trials in neonatology, they were able to show that survival was better in preterm infants who were kept in incubators that were 4° warmer than in the control infants ( 21 ). Despite these results, it took several years before they were translated into routine care of preterm infants. (As a pediatric resident in the mid-1960s, I remember that it was considered normal for preterm infants to have lower body temperatures than term infants.)
Subsequently, the factors that affect the equation of heat loss versus heat production were elucidated ( 22 ). The importance of radiant heat loss led to the introduction of radiant warmers, both in the delivery room and in premature nurseries. It was also noted that an important component of heat production was the presence of brown fat ( 23 ). It was demonstrated that not all infants of low birth weight (LBW) were born preterm but might be small for gestational age or experience IUGR ( 24 ). Some were found to have difficulty maintaining their body temperature, largely because they lacked brown fat. Because other substrate was used to produce heat, they also developed low blood glucose levels. For different-sized infants, at different postnatal ages, a range of temperatures, called the neutral thermal environment ( 25 ), was found to minimize energy expenditure.
Another important advantage of the modern incubator is improved visibility. When clear plastic incubators were introduced in the late 1940s, “Nurses and doctors stared at the naked babies as if they were seeing them for the first time. Naked infants were examined more completely, observed more closely, and treated more actively than ever before” ( 18 ).
Keeping infants warm is a basic necessity; so, too, is providing appropriate nutrition.
Infant formula.
After the discovery of the chemical composition of milk in the 1890s, various percentages of protein, fat, and carbohydrate were used to prepare feeding, called “formula” feeds; the feeding that approximated human milk was introduced in the 1920s. Evaporated cow milk was the basic milk product used, to which was added carbohydrates such as Karo syrup, both for term and preterm infants. To supplement breast milk, DaFoe, in caring for the Dionne quintuplets, used a preparation of evaporated milk, water, and corn syrup, to which a few drops of rum were added ( 26 ).
The work of Levine and Gordon in the early 1940s documented that preterm infants who were fed formulas with increased protein, calcium, phosphorus, and sodium and decreased saturated fats grew more rapidly. However, high protein intake resulted in fluid retention, azotemia, and metabolic acidosis. The introduction of whey-predominant formulas for the preterm infant in the early 1980s resolved many of these problems ( 27 ).
Nevertheless, “breast is best” is the motto that most neonatologists promote today ( 28 ). The composition of human milk expressed by mothers who have delivered prematurely was found to differ from that produced at term and is more appropriate for preterm infants, even though supplements are required to increase caloric strength and protein, sodium, calcium, and phosphorus ( 29 ).
Techniques for providing nutrition.
Until the preterm neonate became “a patient to be cared for,” attempts at providing adequate nutrition were limited. Gavage feeding using soft red rubber catheters was reported as early as 1851 by Marchant of Charenton, according to Budin ( 30 ), and polyethylene tubes were introduced in the 1950s ( 31 ).
The difficulty in preterm infants is the immaturity of the gastrointestinal tract, especially with immature motility patterns and delayed transit. Particularly, in the presence of edema and difficulties with respiration, many preterm infants in the United States were deprived of all nutrition and were frequently starved for up to 72 h or even longer. In hindsight, it is hard to understand the logic behind this approach. Many European physicians, especially Ylppö, were critical of the idea. Early feeding of preterm infants with breast milk was promoted in Oxford as early as 1964 ( 32 ). As difficulties with hypoglycemia and hyperbilirubinemia were better appreciated in these preterm infants, the need for the early provision of nutrition became more apparent in the United States ( 33 ).
Difficulties in providing enteral nutrition stimulated the introduction of i.v. feeding, a major advance. Another important advance was the development of microinfusion pumps, now able to deliver increments as small as 0.1 mL/h. This facilitated the accurate administration of i.v. fluids to extremely preterm infants.
Initially, peripheral venous nutrition was primarily in the form of glucose. Numerous investigators attempted to infuse protein hydrolysates to the preterm or postsurgical infant, but it was Dudrick and Wilmore who worked initially with laboratory animals in Rhoads's Department and developed the basis by which high caloric i.v. preparations, with appropriate nitrogen concentrations, could be infused into large-caliber vessels ( 34 , 35 ).
These techniques were rapidly applied to the care of the low birth weight infant ( 36 , 37 ). At first, the protein was provided by casein or fibrin hydrolysates, but complications such as metabolic acidosis, hyperammonemia, and azotemia resulted. Newer amino acid preparations were developed, and these complications were almost completely eliminated ( 38 ). The addition of trace elements and vitamins enhanced the i.v. cocktail, and after a great deal of difficulty in identifying an appropriate i.v. lipid preparation, this, too, was added to the nutritional support of the preterm infant ( 39 ).
Initially, these preparations had to be infused into major veins and even the umbilical arteries. Peripherally inserted central venous catheters have been used with increasing frequency in recent years. Peripheral venous lines have also been used with lowered concentration of glucose (10–12%) for short-term nutritional support.
Over time, it was learned that there are limitations to the amount that can be tolerated, usually 4 g · kg −1 · d −1 of amino acid and 3.5 g · kg −1 · d −1 of lipid ( 40 , 41 ). Currently, the major difficulties that we have with total parenteral nutrition in these infants is our inability to provide adequate calcium and phosphorus, and also the development of total parenteral nutrition–induced cholestasis.
Recently, trophic feeds (minimal enteral feeds) have been advocated for the very immature infant to enhance the more rapid development of gastrointestinal function. Early reports of the use of this technique have been associated with a decreased incidence of both cholestasis and nosocomial infections ( 42 ).
Growth norms.
Several groups published growth curves to indicate the anticipated measurements for birth weight, length, and head circumference at various gestational ages. Some of these data were gathered at high altitude ( 43 ) and others at sea level ( 44 ) and allowed for the introduction of the concept of small-, appropriate-, and large-for-gestational-age infants ( 45 ).
Disordered fetal growth.
The system of classification of neonates into small, appropriate, or large for gestational age was published in 1967, but the realization that some fetuses were either undergrown or overgrown came several years earlier. Farquhar in Scotland ( 46 ) and Gellis and Hsia ( 47 ) and Cornblath ( 48 ) in the United States had written about the overgrowth of infants of diabetic mothers and recognized that they were prone to a litany of problems, such as hypoglycemia, respiratory distress syndrome (RDS), hyperbilirubinemia, hypocalcemia, hypertrophic cardiomyopathy, etc., when mothers were poorly controlled.
Gruenwald ( 49 ) drew attention to the idea that undergrowth might be the result of placental insufficiency, and Warkany et al. ( 50 ) probably introduced the term “intrauterine growth retardation,” which recently was modified as “intrauterine growth restriction” because parents are frequently alarmed by the word “retardation.” Adding to the confusion are other terms such as fetal malnutrition, dysmaturity, hypotrophy, and light or small for dates. This disorder has its own set of immediate problems, such as fetal distress, hypoglycemia, hyperviscosity, etc. but may also have life-long consequences on health, such as hypertension and diabetes; this is known as the “Barker hypothesis” ( 51 ). Although careful control of diabetic mothers during pregnancy has decreased the number of classical infants, the ability to affect the outcome of IUGR fetuses has been more limited, probably because of multifactorial cause.
Postmaturity.
Before birth weight/gestational age classification was introduced, it was recognized that in addition to many premature infants, some were born postmature, usually >42 wk gestation, and were distinguishable by features such as meconium staining of the umbilicus and fingernails and deep creases on the feet. Stewart Clifford provided a detailed discussion of such infants ( 52 ). Like other dysmature infants, postmaturity was associated with problems linked to hypoxemia and undernutrition. Recently, obstetric management resulted in an aggressive approach to prevent postterm delivery ( 53 ).
RESPIRATORY SUPPORT
Until the mid-1960s, the primary support for respiratory function was the provision of supplementary oxygen. This simple therapy proved to have devastating consequences when the philosophy of “if a little is good, a lot should be better” was espoused and many preterm infants developed retrolental fibroplasia, now called retinopathy of prematurity (ROP).
In an era when it was difficult to measure oxygen tension in the blood, the only way to determine whether an infant was adequately oxygenated was to observe color. If the infant was cyanotic, then oxygen was administered to relieve cyanosis but often in concentrations that produced superoxygenation, it was later determined. Cyanosis in adults is usually clinically apparent when ∼ 5 g/100 mL of deoxygenated (reduced) Hb is circulating. It was shown in neonates that cyanosis was evident when 3 to 4 g/100 mL of deoxygenated Hb was present ( 54 ).
The ability to measure arterial oxygen tension made a huge difference in our ability to manage oxygen therapy. Blood samples of ∼ 2 mL were needed, but by the end of the 1960s, sample size was down to 0.5 mL and subsequently to 0.2–0.3 mL. Because of the association of ROP with the liberal use of oxygen, there was proscription of the use of 100% oxygen. Incubators of that era were designed so that no more than 40% oxygen could be delivered, unless a baffle on the back of the incubator were closed. When this was done, it literally raised a red flag to indicate that dangerous amounts of oxygen were used. The pendulum had swung the other way, and infants undoubtedly died because they were deprived of adequate amounts of oxygen ( 18 ).
The physiologic studies of Joseph Barcroft, Donald Barron, Clement Smith, John Lind, Geoffrey Dawes, and others showed that the fetal animal and, presumably, the human had a much lower circulating Po 2 than the human newborn. This had specific import for the infant who was born very preterm (<32 wk), because these infants would not have been exposed to even normal levels of Po 2 had they remained in utero . The specific cause of ROP is probably multifactorial, but the generation of oxygen-free radicals almost certainly is contributory ( 55 ).
Resuscitation in the delivery room.
It is now hard to appreciate the benign neglect that occurred in most delivery rooms in the world until the late 1950s. Earlier, Virginia Apgar had reported her scoring system and in 1958 ( 56 ) proposed that someone other than the delivering obstetrician or midwife should concern him- or herself with the infant or infants. She suggested that the infant should be evaluated using five parameters—heart rate, respiration, reflex activity, tone, and color—within the first minute, and, if necessary, intervention to improve the situation should occur before reevaluation of the infant at 5 min ( 56 ). Although the method has occasionally been questioned, it remains a very valuable tool in neonatal assessment around the world ( 57 ). It should be emphasized that Dr. Apgar never claimed that her score was predictive of long-term outcome, and it should not be used for this purpose.
Virginia Apgar may have been the first person to insert an umbilical artery catheter for the purpose of measuring arterial blood gases ( 58 ). This technique was certainly key and improved our ability to obtain better results with assisted ventilation.
During the past few years, the need for 100% oxygen in the delivery room has been questioned, because many infants can be resuscitated successfully in room air ( 59 ). This idea has not been universally accepted at the time of writing. What has been accepted is the role of bag-and-mask ventilation or endotracheal intubation and assisted ventilation when an infant fails to establish spontaneous respiration. Both of these techniques followed logically from evaluation of the Apgar scores and replaced other methods that were considered useful but that had marginal utility. When I graduated from medical school in Edinburgh, Scotland, in 1961, the approved method of resuscitation was intragastric oxygen. In Glasgow, hyperbaric oxygen was proposed for this purpose ( 60 ), although it sometimes took approximately half an hour to place the infant in the device!
One specific aspect of neonatal resuscitation is the approach to prevent meconium aspiration syndrome ( 61 ). Although not all cases can be prevented, suctioning of the oropharynx with the head on the perineum, followed by prophylactic endotracheal intubation and suctioning became an early example of obstetrician and pediatrician collaboration ( 62 ).
In the past 20 y, the establishment of a national resuscitation program in the United States codified neonatal resuscitation in a way that could be taught to thousands of physicians and nurses ( 63 ). The program has been exported to other countries, and although it has had wide acceptance and application, it may be replaced by more sophisticated methods in the future.
Despite the widespread improvement in neonatal resuscitation, intrapartum asphyxia continues to be a problem. It was hoped that the use of fetal heart rate monitoring and fetal scalp sampling, to evaluate fetal blood pH, would eliminate this concern, but it has not been the case. It was also assumed that careful fetal evaluation by the obstetrician would decrease the incidence of cerebral palsy. This has been shown to be a false assumption, with few cases (maybe 15%) of cerebral palsy attributable to intrapartum events ( 64 ).
Intrapartum asphyxia was found usually to be the result of either poor uteroplacental blood flow ( e.g. with hypertensive disorders) or a sudden hypoxemic event ( e.g. prolapsed umbilical cord, acute placental abruption). The important studies of asphyxia in primates performed by Dr. William Windle and later by Ronald Meyers and Albert Brann deserve special mention. The situations above roughly equate to partial prolonged asphyxia or total acute asphyxia evaluated in the primate ( 65 ). Because of the poor outcomes after a prolonged asphyxial insult, methods to protect against such injury have been sought. Recently, this has stimulated attempts to decrease brain metabolism by using moderate systemic hypothermia or localized brain hypothermia. However, the window of opportunity for this therapy is open for only a short time ( 66 ).
Assisted ventilation.
It was not until the mid-1960s that attempts were made to provide continuous respiratory support, using mechanical ventilation, for infants with severe respiratory disease. At that time, most preterm infants who died after several days were found to have histologic evidence of hyaline membranes in the lungs at postmortem examination. They were considered to have hyaline membrane disease (HMD). Mary Ellen Avery and Jere Mead showed in 1959 that this disorder was linked to a deficiency of surfactant in lung fluid ( 67 ). At approximately the same time, the alternative hypothesis was proposed that pulmonary hypoperfusion might be the determining factor in development of hyaline membranes ( 68 ).
Because HMD was the apparent cause of death of the infant son of President John F. Kennedy, research money became available to investigate the cause and support the management of the disorder. Not all infants with the disease died but often demonstrated spontaneous improvement after increasing severity of illness for 2 or 3 d. At approximately this time, Robert Usher, in Montreal, showed in a randomized, controlled trial that RDS mortality could be reduced by using a constant infusion of i.v. glucose and bicarbonate to minimize hyperkalemia and acidosis ( 69 ). In the 1960s, this was known as “the Usher regime.”
Portable x-ray machines became available and contributed to the more accurate assessment and aggressive management of respiratory disorders of the neonate. Earlier, infants were considered too sick to have an x-ray. Now, the machine came to the infant, instead of taking the infant to the machine.
A national consensus conference determined that the term RDS was probably more appropriate than HMD, because HMD was a pathologic diagnosis. However, this consensus was a small majority. Because of the uncertain cause, “idiopathic” was frequently placed before RDS (hence, IRDS).
In the mid-1960s, a group of investigators in several different centers began to adapt mechanical ventilators, used for adults, to assist ventilation of infants with RDS with the hope that they could be helped through the critical first days, until spontaneous resolution might occur. In the beginning, only the sickest infants were tried on assisted ventilation, with variable success. Many times it was possible to produce improvement for several days, only to have the infant succumb days later. In the early days of mechanical ventilation, both positive-pressure ventilators and negative-pressure ventilators were used.
I was fortunate to spend 3 mo in 1969 at the Port Royal–Baudelocque Center in Paris, which was headed by Prof. Alex Minkowski. Early in my stay, I observed a conference on assisted ventilation, the proceedings of which were later published in Biology of the Neonate (the journal established by Prof. Minkowski as Biologia Neonatorum ) ( 70 ). The big question was whether it was appropriate to continue to push forward with assisted ventilation in light of technical difficulties—the negative-pressure ventilator was almost impossible to use in infants who weighed <1500 g—and other complications such as bronchopulmonary dysplasia (BPD), which had already been described with positive-pressure ventilation ( 71 ). The answer now is obvious, but there was a good deal of soul searching at the time.
Two specific problems related to assisted ventilation deserve comment: BPD and pulmonary interstitial emphysema. BPD was first described in 1967 ( 71 ) and was sometimes called “respirator lung” ( 70 ). It was subsequently recognized that this was not exclusively oxygen toxicity but seemed to be caused by the combination of barotrauma (pressure), oxygen, and the duration of exposure of the immature lung to both ( 72 ). In the 1970s and 1980s, prolonged hospitalization as a result of the consequences of lung injury was common. Cystic change and marked hyperinflation were not uncommon and frequently led to cor pulmonale and death. Since the advent of surfactant treatment for RDS, BPD is a much more benign disorder. It is now commonly referred to as chronic lung disease of prematurity, with radiographic features that are much less dramatic but are accompanied by prolonged oxygen dependence ( 73 ). However, this is usually for several weeks beyond term gestation, whereas it was formerly for several months.
Before the introduction of surfactant therapy, assisted ventilation was frequently accompanied by air leaks, usually pneumothorax and pneumomediastinum, which now can be detected with high-intensity fiberoptic transillumination, introduced in the 1970s ( 74 , 75 ), allowing emergency intervention. As our ability to ventilate extremely preterm infants improved and surfactant was more widely used, the incidence of pneumothorax decreased ( 76 ). However, pulmonary interstitial emphysema remains a problem and can be extremely difficult to treat. This situation seems to be where high-frequency ventilation has been most successful (compared with conventional ventilation) ( 77 ).
Continuous distending pressure.
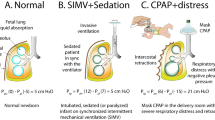
From the observations of Avery and Mead and other studies, it was well recognized that atelectasis was a major component of RDS. The group at the University of California, San Francisco, proposed that RDS be called progressive pulmonary atelectasis to describe more accurately the pathophysiology of the disorder. Not only was assisted ventilation enhanced by using positive end expiratory pressure (PEEP), but also the principle was used de novo early in the course of RDS by the group at the University of California, San Francisco ( 78 ). They called this continuous positive airway pressure (CPAP), although the principle is the same as PEEP. Some infants (neonates) with RDS could be treated with CPAP alone, although many went on to need assisted ventilation with PEEP.
Early in the application of CPAP, attempts were made to apply it with either a face mask or a head hood, but it was most successful using an endotracheal tube. Soon, a group in Cleveland used a nasal device to provide CPAP ( 79 ); nasal prongs have persisted as the preferred method of application since then, although there have been variations.
When negative-pressure ventilators were used, there was also the capability to provide continuous negative pressure. Several attempts were made to provide free-standing negative-pressure devices (a body cuirass), but they have not caught on, despite that avoiding endotracheal intubation seems to be a worthy goal. The use of continuous distending pressure has been the mainstay of all subsequent ventilatory techniques, including pressure-generated, volume-generated, or high-frequency ventilation.
Assessing fetal lung maturity.
Fetal assessment is generally the domain of the obstetrician, particularly the maternal–fetal specialist, although the neonatologist has an interest in how the fetus is evaluated and treated. Louis Gluck, a pioneer in neonatology, devised a method of assessing lung maturity in the fetus using the ratio of lecithin, phosphatidyl choline, to sphingomyelin (L/S) in amniotic fluid ( 80 ). This advance resulted from earlier work that demonstrated that fetal lung fluid in the airways contributed to amniotic fluid, rather than the reverse ( 81 ). It was not uncommon, before the introduction of measurements of the L/S ratio, for obstetricians to deliver infants who were only slightly preterm and went on to develop severe RDS.
An L/S ratio of >2:1 usually signifies mature lungs. Subsequently, measurements of phosphatidyl glycerol in amniotic fluid were found to be even more predictive of RDS than the L/S ratio. Infants of diabetic mothers had a delay in lung maturation, which may result from fetal hypersecretion of insulin, blocking the enzyme inductive capability of cortisol in the lung. This may be overcome by careful glucose control ( 82 , 83 ).
Prenatal corticosteroids.
The discovery and application of prenatal (antenatal) corticosteroids was concurrent with assessment of fetal lung maturity. This started in 1972 with the publication of the use of betamethasone to prevent or minimize the severity of RDS ( 84 ). Possibly because it was published in a pediatric journal but also because a subsequent collaborative study published in an obstetric journal provided a less conclusive response ( 85 ), it was several more years before the body of evidence convinced obstetricians to sign on to this remarkably beneficial adjunct in the care of the preterm infant. These data were collected in a consensus conference, published in 1994 ( 86 , 87 ). It was also documented that antenatal betamethasone has a protective effect against the development of intraventricular hemorrhage (IVH) ( 87 , 88 ).
Exogenous surfactant.
After the Avery-Mead discovery of the link between surfactant lack and development of HMD (RDS) [which followed the work of Pattle ( 89 ) and Clements ( 90 )], attempts were made to provide the major component of surfactant, dipalmitoyl phosphatidyl choline, via the endotracheal tube, but these proved disappointing. It was not until 1980 that Fujiwara reported success in human newborns using exogenous liquid surfactant derived from minced calf lung ( 91 ). At that time, others were working on surfactant extracts derived from human amniotic fluid or a synthetic product ( 92 , 93 ); there was also a powdered form of surfactant, with the acronym ALEC, for acute lung expansion compound ( 94 ).
Over the next few years, many nurseries participated in randomized, controlled, clinical trials of different products. In the United States, the two major competitors were Exosurf (a synthetic product produced by Burroughs-Wellcome) and Survanta (derived from calf lung and produced by Ross Laboratories). Although Exosurf received Food and Drug Administration approval first in 1990, Survanta was close behind and rapidly gained greater acceptance because of more rapid improvement. In Europe and Scandinavia, a porcine-derived surfactant, Curosurf, also rapidly gained ascendancy because of its rapid onset of improvement and demonstrable decrease in pulmonary mortality and morbidity ( 95 ).
There are several other competing products, which claim greater efficacy, but the success of all of these animal-derived products seems to be related to the presence of surfactant proteins ( 96 ). The impact of exogenous surfactant on neonatal survival has been enormous ( 97 ).
Cardiopulmonary monitors.
Until the 1960s, the bedside nurse was responsible for taking vital signs at intervals dictated by the severity of illness of the infant. However, when it was recognized that prolonged and frequent apneic episodes might result in long-term consequences, apnea monitors were introduced ( 98 , 99 ). The prototypes were impedance monitors that picked up electrical signals across the chest, but pneumatic mattresses that picked up breathing movements enjoyed brief popularity. These monitors were set to alarm at 15 to 20 s of apnea. Continuous heart rate monitors also became available with a needle pointing to a number. Subsequently, cardiopulmonary monitors with a waveform displayed on a screen became standard equipment in intensive care, or special care, nurseries, as an extension of the nurse. Blood pressure monitoring was added later.
Oxygen saturation monitors.
Intermittent oxygen tension measurements using microsamples of blood gave way to transcutaneous oxygen monitoring in the mid-1970s ( 100 ), a completely different view of neonatal oxygenation that provided a continuous recording. However, measurement of transcutaneous oxygen requires a heated electrode, which needs to be moved frequently to avoid skin injury, especially in the very preterm infant.
In the 1980s, pulse oximetry was introduced to neonatology ( 101 ) and rapidly gained popularity because it used a light sensor that could be wrapped around the foot or the hand to detect oxygen saturation. Because there was no heating device, the position did not need to be changed frequently. However, when the pulse was not being detected, the monitor would alarm, and this occurred frequently when the infant was moving. This problem now seems to be solved with some devices.
Apnea, sudden infant death syndrome, and methylxanthines.
At the end of the 1960s, it was agreed that if attacks of apnea were frequent and prolonged, then hypoxic brain damage was likely to follow. In the early 1970s, Kuzemko wondered whether aminophylline might ameliorate this problem, because it was known to act on the respiratory center ( 102 ). Subsequently, other studies showed the value of aminophylline, as well as noting the interconversion of theophylline with caffeine. Caffeine then was used to treat apnea ( 103 ), and because it can be given once a day, it has gained greater acceptance.
Also in the early 1970s, the hypothesis was generated that apnea was the precursor of sudden infant death syndrome (SIDS). In some centers, this created a whole new industry of home apnea monitoring, which was based on parental fear of SIDS. This was categorized a few years ago as a fraud perpetrated on the public ( 104 ). The “Back to Sleep” campaign has been much more successful in preventing SIDS ( 105 ).
CARDIOPULMONARY SUPPORT
It is not always possible to consider the lungs in isolation, and there are several other innovations that have had an impact on the outcome of neonates, both preterm and term, that are more concerned with the circulatory system but inevitably have an impact on the pulmonary system.
Patent ductus arteriosus management.
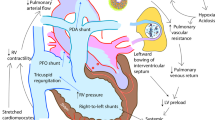
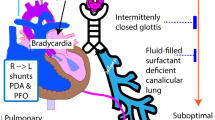
With improvements in management of RDS, many preterm infants had rapid changes in the dynamics of pulmonary blood flow, which resulted in left-to-right shunting through a patent ductus arteriosus (PDA). Most of these infants were considered too small or unstable to attempt surgical ligation of the PDA. Although this attitude has changed in recent years (with surgical ligation now being done in the corners of many NICUs), the discovery that the PDA could be closed pharmacologically with indomethacin ( 106 , 107 ) had a major impact on the practice of neonatology.
In some circumstances, the opposite strategy is used. Prostaglandin E has been used since 1975 to maintain the patency of the ductus arteriosus in cases of cyanotic congenital heart disease ( 108 ).
Blood pressure support.
Methods of measuring systemic blood pressure in the neonate were crude until the 1970s, when umbilical arterial catheters were frequently inserted. This gave direct access to arterial blood pressure, and the normal range of blood pressure could be determined ( 109 ). Subsequently, noninvasive methods became available, which provided measurements comparable to those obtained directly. With these devices, combined with clinical evaluation of peripheral blood flow, much greater attention was given to maintenance of both adequate circulatory blood volume and blood pressure. The use of colloid and crystalloid increased markedly ( 110 ), as did the use of pressor agents, such as dopamine and dobutamine ( 111 ).
Persistent pulmonary hypertension management.
With the advances in measurement of systemic blood pressure came a more sophisticated understanding of pulmonary blood pressure. Under certain circumstances, most notably with asphyxia and/or meconium aspiration syndrome, the syndrome called “persistent fetal circulation” was described ( 112 ), with persistence of right-to-left shunting at the ductus arteriosus and foramen ovale seen in utero . This was really a misnomer and should have been called “persistent transitional circulation,” because the placenta was no longer in the circuit. The problem is encountered most frequently in term or postterm infants. This now is usually described as persistent pulmonary hypertension of the newborn (PPHN). There were two approaches adopted to try to correct this: either increase systemic blood pressure or decrease pulmonary blood pressure. For many years, tolazoline (Priscoline) was used to decrease pulmonary blood pressure, sometimes with dramatic success ( 113 ), but this agent had a tendency to cause a decrease in systemic blood pressure at the same time. To counter this, pressors were used. It was also noted that the condition tended to worsen with agitation or crying, so sedative/analgesia drugs were introduced and even paralysis. Thus began the era of polypharmacy for this condition.
At least two other adjuncts to therapy that have had variable acceptance were described. First, it was noted that hyperventilation seemed to produce improvement, and it was not clear for a while whether this was because of decreased CO 2 or increased pH. Subsequently, it was shown convincingly that increased pH (alkalemia) was the important component ( 114 ). Second, an agent used in obstetrics for muscle relaxation was used in the neonate. This is magnesium sulfate, which seems to have a selective effect on the pulmonary arterioles ( 115 ).
Despite any or all of these interventions, the infant went on to die in many situations. This was the result of peripheral extension of the arterial/arteriolar muscle into the capillaries ( 116 ). The most likely stimulus seemed to be chronic intrauterine hypoxemia.
Two other modes of therapy had a significant impact on the management of PPHN: 1 ) inhaled nitric oxide (iNO) and 2 ) extracorporeal membrane oxygenation (ECMO). One of the more intriguing discoveries in recent years was that iNO, acting as a selective pulmonary vasodilator, could produce marked benefit in neonates with PPHN ( 117 , 118 ). The early reports used considerably higher concentrations than those generally used today. The ability to deliver a therapeutic agent via inhalation directly to the site of the problem meant that undesirable side effects are less likely. Earlier intervention with iNO has contributed to a decrease in the number of infants who require ECMO.
When all else fails in the management of PPHN, there is always the possibility of achieving success with ECMO. This technique involves cannulation of major blood vessels, usually the carotid artery and jugular vein, and requires very careful evaluation of coagulation status. The underlying lung pathology may resolve while the pulmonary circulation is largely bypassed.
When first introduced, the studies of ECMO generated a good deal of controversy because of the statistical methods used at the University of Michigan ( 119 ) and a somewhat different but equally contentious method at Children's Hospital, Boston ( 120 ). Because of the controversy engendered, it was possible for researchers in the United Kingdom to maintain equipoise and undertake a randomized, controlled trial that confirmed the value of ECMO ( 121 ). Most centers do not undertake this form of treatment in infants who weigh <2 kg, and it is recommended that it be limited to centers that perform the procedure frequently to maintain the skills required.
Echocardiography.
While considerable advances were attributed to the introduction of cardiac catheterization in the mid-20th century, the ability to detect structural abnormalities with the use of cardiac ultrasound, echocardiography, revolutionized pediatric and neonatal cardiology ( 122 ). Using Doppler techniques, the direction of blood flow could be detected. This made the detection of PDA and intracardiac shunting secondary to PPHN relatively simple.
Other advances in neonatal echocardiography are beyond the scope of this article. However, it should be mentioned that fetal echocardiography has assumed increasing importance in the past decade, allowing early diagnosis of congenital heart disease to be made and delivery at a cardiac center to be facilitated.
NEONATAL INFECTION
The start of the antibiotic era coincided with the beginning of modern neonatology. Before the introduction of penicillin, the most important organisms causing neonatal sepsis and meningitis were Streptococcus pyogenes (group A Streptococcus ), Staphylococcus aureus , and Escherichia coli . The Staphylococcus rapidly developed resistance to penicillin and created an extremely problematic situation in most hospital nurseries. The “cloud” infant was described ( 123 ), cultures from many sites were sent, and cohort nurseries were established ( 124 ). Hexachlorophene was widely used to suppress the organism but proved to have its own hazard in premature infants, causing cystic lesions of the brain ( 125 ). When methicillin was discovered, which was effective in treating S. aureus , there was a great sense of relief.
The difficulty of making a definitive diagnosis of neonatal sepsis or meningitis early in its course has been recognized for decades. This limits our ability to restrict the use of antibiotics to neonates with known infection. In the 1970s, more attention was paid to interpretation of the white blood cell count and differential, as well as acute-phase proteins ( 126 ). C-reactive protein was shown in Sweden to be helpful in diagnosis as long ago as 1974 ( 127 ) but was not widely embraced in the United States. The immature to total neutrophil ratio also proved to be a useful adjunct ( 128 , 129 ). Until the present time, no single test has proved reliable in making an early diagnosis of sepsis, although this may be about to change if the PCR technique is used to identify bacteria ( 130 ).
In the early 1970s, S. agalactiae (group B Streptococcus ) became a serious concern across the country, although isolated reports had been published earlier ( 131 ). “Early” and “late” forms of the disease were described ( 132 ). Only recently has obstetric practice changed to minimize the effect of this organism ( 133 ). However, as one bacterial organism declines, there is always another to take its place. E. coli has remained a fairly constant threat, and several multicenter trials were organized to evaluate the management of Gram-negative bacillary neonatal meningitis ( 134 ). Antibiotic-resistant bacteria remain a continuing concern in all nurseries. In the very low birth weight (VLBW) infant, coagulase-negative staphylococci play an important role ( 135 ) and have limited sensitivity—most are still sensitive to vancomycin–but fungal organisms, such as Candida albicans and Malassezia furfur , may be very difficult to eradicate ( 136 ).
Intrauterine infection with nonbacterial organisms was well described in the 1960s, when the expanded congenital rubella syndrome was described ( 137 ) and investigation with TORCH titers became common. This acronym stood for toxoplasmosis, other, rubella, cytomegalovirus, and herpes simplex; chief among “other” was congenital syphilis ( 138 , 139 ). Later, we were introduced to parvovirus B 19 , which can cause profound anemia and hydrops of the fetus ( 140 ), and on a global basis, perinatal transmission of HIV-1 has had a devastating effect on millions of infants who became HIV positive or acquired AIDS. Although perinatal transmission may now be prevented in many cases, availability of appropriate drug therapy remains a problem ( 141 ).
OTHER IMPORTANT PROBLEMS OF PREMATURITY
With advancing knowledge, the premature infant was no longer labeled a weakling but was evaluated carefully and found to have a variety of definable problems, many of which are now treatable. Several important problems deserve further comment.
Necrotizing enterocolitis.
Necrotizing enterocolitis continues to be a major complication that develops in the most immature infants. Mortality was extremely high until the early 1970s, at which time a group of physicians at the University of Washington noted that early recognition and aggressive medical and surgical management could significantly reduce mortality ( 142 ). In addition, staging of the disease process has allowed better evaluation of the various approaches to diagnosis and care. Pediatric surgeons are currently conducting a randomized, controlled trial of immediate exploratory laparotomy versus simple drainage followed by later exploration. Despite a better understanding of the disorder and that feeding with breast milk provides some protection, necrotizing enterocolitis still affects 7–10% of infants with birth weight <1500 g and causes increased morbidity and prolonged hospitalization ( 143 ).
IVH/periventricular hemorrhage.
Brain imaging was introduced into neonatology in the mid-1970s ( 144 ). This was initially with computed tomography, which revolutionized our ability to determine whether intracranial hemorrhage had occurred. In 1979, the first reports of ultrasound imaging of the brain were published ( 145 ). This was even more exciting, because it could be done at the bedside, whereas computed tomography scanning required moving the infant to the machine (still not an easy task when an infant is on assisted ventilation). Particularly with head ultrasound, it was possible to confirm rapidly (or refute) the clinical suspicion of IVH. Too often, clinical impressions proved to be incorrect, and many sick infants with falling hematocrits were found not to have intracranial hemorrhage ( 146 ).
Cranial ultrasound allowed better understanding of the pathophysiology of IVH/periventricular hemorrhage (PVH), but it remains a comparatively common problem in the extremely preterm infant, especially before 29 wk gestation. The incidence of grade 3 (hemorrhage dilating the ventricles) and grade 4 (extension of hemorrhage into the brain parenchyma) IVH/PVH has decreased significantly in the past 20 y ( 147 ), particularly with the increased use of prenatal corticosteroids ( 87 , 88 ), improved recognition and treatment of chorioamnionitis, improved obstetric management, and postnatal use of indomethacin ( 148 ). Nevertheless, up to 15% of VLBW infants (<1500 g) are still affected by IVH/PVH, which is likely to have long-term adverse consequences on neurodevelopmental outcome ( 149 , 150 ).
Whereas sequential head ultrasound scanning is frequently used early in the neonatal course, magnetic resonance imaging, which was introduced approximately a decade afterward, is the preferred technique. Magnetic resonance imaging provides greater definition and clearly delineates white and grey matter. Adding spectroscopy to the imaging yields more information about function and structure ( 151 ).
Formerly called retrolental fibroplasia because in its most severe form the retina may be scarred or even become detached, ROP causes considerable heartache. The role of the misuse of oxygen in its cause has been well documented by William Silverman ( 18 ), but this is a complex disorder that is still being elucidated ( 152 ). The disorder cannot be evaluated until the blood vessels of the retina are of a certain maturity; examinations usually start at 6 wk after birth, so this is usually the last of the problems of prematurity to declare itself. In the sickest of preterm infants (<29 wk gestation), it hangs over parents like the sword of Damocles. It is usually not possible to provide reassurance that the eyes have been spared until close-to-term gestation.
At one time, severe stages of ROP inevitably resulted in blindness, but in the past 15 y, there has been considerably more optimism. In 1988, the first ray of hope was provided by a preliminary report of improved outcome in severe ROP with the use of cryotherapy ( 153 ). Subsequently, the more usual approach is to ablate the proliferating blood vessels with laser therapy, with or without cryotherapy. Although this does not guarantee normal vision, it is more likely that there will be a successful outcome.
NEONATAL SURVIVAL
Although neonatal mortality is defined as deaths that occur before 28 d after birth, many authors in recent years have considered that all deaths before discharge from the nursery should be considered in that statistic. Thus, survival to discharge may be a more appropriate way of looking at things. Parents, before delivery at an early gestational age, want to know two things: 1 ) what are the chances that the infant will survive; and 2 ) if the infant survives, what are the chances of neurodevelopmental disability?
As recently as the mid-1960s, extremely low birth weight (ELBW; <1000 g) infants had a survival rate of ∼ 5% ( i.e. the mortality rate was 95%) ( 154 ); by 2000, the survival rate in neonates with birth weight 901–1000 g was 95%. In 1960, infants who were born at <28 wk gestation were considered “previable.” Today, >50% of infants who are born at 24 wk gestation survive ( 149 ).
The gestational age at which 50% of neonates survived decreased from 29 wk in 1960 to 24 wk by the early 1990s. Although the long-term outcome for VLBW (<1500 g) survivors was poor in the 1960s ( 155 ), infants with birth weights of 1000–1500 g now do well. However, the number of infants with disabilities has stayed approximately the same because of increased survival at lower gestational ages. In survivors who are born at 23–24 wk gestation, major disabilities occur in 20% and mild to moderate disabilities occur in an additional 20–30% ( 150 ). This means that 50% of such infants may be normal. Unfortunately, it is difficult to predict accurately into which half an infant will fall.
Neonatal survival and/or mortality is strongly linked to the number of premature or LBW infants born. Intensive efforts to decrease the incidence of prematurity using a number of different strategies have had very little impact on the problem. In the United States, the incidence of prematurity has increased in recent years. This is largely due to an increase in the number of multiple births (twins, triplets, and higher), which are more likely to result in a preterm delivery. Approximately 25 y ago, multiple births accounted for 1.8% of all pregnancies, whereas currently, the proportion is ∼ 2.5%.
This increase in multiple births is the result of advances in assisted reproduction techniques such as pharmacologic induction of ovulation, standard in vitro fertilization, and intracytoplasmic sperm injection, which now are commonplace. However, even with certain safeguards ( e.g. implanting only two embryos), the risk for multiple births is high ( 156 ). Not only that, a recent study from Australia ( 157 ) showed that there may be an increased risk for birth defects. Assisted reproductive techniques accounted for >40% of multiple pregnancies beyond simple twinning and for many more LBW and VLBW infants ( 158 ).
REGIONALIZATION/DEREGIONALIZATION
In the late 1960s and early 1970s, many areas of medicine were the beneficiaries of the Regional Medical Programs initiative started in 1965. In some areas, neonatal care benefited from this initiative—although heart disease, cancer, and stroke were targeted—and the success of these programs resulted in wider dissemination of the concept. In addition, comparative data indicated that mortality and morbidity were higher in infants who were transferred to centers after birth in comparison with those who were born at the center ( 159 ). When mothers were transferred to the center to deliver, the data showed that such maternal–fetal transfers had mortality and morbidity that was similar to those who originally planned to deliver at the center ( 160 ).
One of the prime movers in this movement was Joseph Butterfield, along with Jerold Lucey and others. In October 1970, an ad hoc committee met in Denver. The group composed a policy statement on regionalization and/or centralization of perinatal care that was endorsed by the American Medical Association House of Delegates in August 1971 ( 161 ).
When NICUs were first introduced, only the fittest infants who were born in community hospitals were able to survive the trip to the regional center. However, this era was followed by concentration on improving the transport of neonates ( 162 ). Such a system was necessary when there were limited resources of equipment and personnel. However, as more and more people went into this new specialty of neonatology, any hospital with aspirations to be a full-service provider decided that it would build a NICU. This resulted in a very competitive climate in some areas, where the number of NICU beds exceeded the demand. There was some natural attrition for a while, but when survival of small infants improved, it was sometimes difficult for the centers (usually academic centers) to be able to accommodate all of the infants who were eligible for transfer. This further stimulated development of NICUs in nonacademic centers.
In the early days of neonatology, the expertise was largely confined to academic centers, where research was conducted. By the late 1980s, many neonatologists preferred the freedom of providing only clinical care and did not want to worry about competing for grant support to conduct research. Consequently, many of them went private.
A certain amount of deregionalization has occurred, which was possible as a result of the expanding numbers of physicians, nurses, and respiratory therapists. In addition, in many areas, having an infant many miles away from where the parents live created an unnecessary burden, especially when appropriate care could be provided closer to home. This resulted in attempts to back-transfer infants when the acute phase of their illness resolved. This is not always what parents want but has become an option for neonates who require an extended hospital stay, usually the very preterm infant.
The concept of concentrating the sickest patients in regional centers persists, despite the establishment of nurseries that are capable of providing a high level of care in community hospitals. For instance, in California, the state designates four levels of care for nurseries. Level 3 is a community NICU, which may be capable of providing assisted ventilation but would not normally perform neonatal surgery or have a large number of consultative services available. Level 4 is a regional NICU, to which community NICUs relate. These NICUs provide the complete range of consultative services—pediatric surgery, cardiology, neurology, ophthalmology, genetics, endocrinology, etc.—and are most commonly affiliated with an academic center.
Although neonatal transfer remains an option and is usually accomplished safely with the use of specialized teams that are trained for this purpose, it is still considered desirable for most extremely preterm fetuses, particularly <30 wk gestation, to be transported in utero for delivery at the regional center ( 163 ). With increased detection of congenital malformations using prenatal ultrasound, it is also usually the case that such infants, who are likely to require surgery, will be delivered at a regional center. In this way, a multidisciplinary approach can be coordinated more easily. All of this has improved the care provided to mothers and infants in a region.
Home birth movement.
The home birth movement could be considered as an extreme extension of deregionalization. In the mid-1970s, there was an upsurge in the belief that the most natural place to deliver was at home. This movement was led by lay midwives, rather than nurse midwives, many of whom were unwilling to acknowledge the risks inherent in delivering infants at home. To counter this movement, a number of hospitals introduced “alternative birth centers” with a more home-like environment for low-risk patients ( 164 ). Recently, the pendulum seems to have swung back again, with the realization that emergencies cannot be dealt with adequately at home.
Perinatal outreach education.
To make a regional program work effectively, there needs to be a close collaboration among maternal-fetal medicine physicians, neonatologists, and perinatal nurses. Community obstetricians, pediatricians, and family practitioners need to learn what they can do under certain circumstances to make sure that mothers and infants receive the best care possible. In addition to stabilization techniques, they need to know what is available at the regional center. The most effective way to do this is to interact personally with referring physicians. In most perinatal regions, this is accomplished through Outreach Education ( 165 ). This kind of interdisciplinary collaboration is one of the major changes that has occurred in the past 40 y.
PARENT–INFANT INTERACTION
Although it may not seem to be an obvious advance in neonatology, it is important to understand the change that has occurred in the attitudes of physicians and nurses to the role of parents in the care of their infants. When I was a pediatric resident, I was barely allowed to enter the premature nursery and parents were allowed to view their infants only through a glass partition [see also ( 6 , 18 )]. There was great emphasis placed on the susceptibility to infection of the premature infant.
All of this changed at the end of the 1960s, with an initial study by Barnett et al. ( 166 ) at Stanford, but was subsequently in response to the work of Marshall Klaus and John Kennell, who promoted the idea of “bonding.” Although this was initially concentrated on maternal–infant interaction ( 167 ), it rapidly expanded to include fathers ( 168 ). T. Berry Brazelton published his Behavioral Assessment Scale ( 169 ) and drew our attention to differences in interactions of infants with fathers. With greater involvement in the nursery, parents gradually assumed more responsibility for decisions concerning their infants. The old paternalistic attitudes of physicians were replaced by a team approach that included the parents. Although it is not always possible, because delivery may occur too rapidly, prenatal consultation with both parents by a neonatologist now is the norm when a mother is in preterm labor at 23–25 wk gestation to discuss possible options regarding resuscitative efforts and subsequent management ( 170 ).
Not only were parents encouraged to come to the bedside or incubator-side and touch the infant and hold the infant, if he or she was not attached to too much equipment, but also mothers were encouraged to contribute to the care of their infants by providing expressed breast milk ( 171 ). This aspect of care allowed both parents to feel more engaged with their infants as fathers are frequently the transporters of frozen breast milk for storage. In some developing countries, the concept of “kangaroo” care was introduced ( 172 ) and such skin-to-skin contact now is frequently encouraged in nurseries in the United States ( 173 ).
NEONATAL METABOLIC SCREENING
One well-established procedure at present, which “always seems to have been around” but was introduced <50 y ago, is screening for metabolic disease using blood spots on filter paper. The first test, introduced in Massachusetts, was a test for phenylketonuria, called the Guthrie test ( 174 ). This was initially proposed as a urine test but then applied to blood spots and was based on a bacterial inhibition assay, introduced in 1961 because increased levels of phenylalanine enhance bacterial growth ( 175 ). States rapidly introduced legislation to support the screening of this disorder in all infants at the time of discharge from the hospital (in term infants).
The PKU test, as it was called for many years, had several other inborn errors of metabolism added to the screening over the next decade, such as homocystinuria, maple-syrup urine disease, and galactosemia. Screening for congenital hypothyroidism was introduced in the early 1970s ( 176 ). This was superimposed on the system already in place, at a time when some were questioning the utility of metabolic screening, because most disorders had an incidence of 1:10,000–1:125,000, or more. However, congenital hypothyroidism has an incidence of 1:3000–1:5000.
Several more tests were added to these basic screening tests in different states ( e.g. assessment of biotinidase deficiency, testing for hemoglobinopathies, immunoreactive trypsin for cystic fibrosis) depending on the racial distribution of the population. Recently, many more (but rare) inborn errors of metabolism can be screened using blood spots and the technique of tandem mass spectrometry ( 177 ). Unfortunately, the current economic climate has limited state support for this technique in some states.
Over the past 40 y, there has been an enormous change in the cause of severe hyperbilirubinemia, manifest most commonly in the neonate as profound jaundice. Whereas 40 y ago, Rhesus incompatibility was a major problem, resulting in the need to perform large numbers of exchange transfusions to prevent the development of kernicterus, it now has been virtually eradicated ( 178 ). Consequently, many pediatric residents and some fellows in neonatology finish their training without having performed an exchange transfusion, almost incomprehensible to the older generation!
Globally, glucose-6-phosphate dehydrogenase deficiency probably contributes the largest proportion of infants with severe hyperbilirubinemia. Studies conducted over more than a decade, particularly in Greece, indicate that tin-mesoporphyrin, which is a potent inhibitor of heme oxygenase, can be used as an alternative to exchange transfusion or as prophylaxis against severe hyperbilirubinemia ( 179 ).
In the preterm infant and others, phototherapy is the mainstay of treatment. Early discharge of term infants has become the norm and makes the task of detecting and preventing severe hyperbilirubinemia more difficult ( 180 ). One adjunct that should facilitate detection is the use of transcutaneous bilirubinometry, first introduced in Japan ( 181 ), which is a screening tool and lessens the need for blood tests. There now are published norms for age-specific levels of total serum bilirubin, hour by hour from 18 to 168 h after birth ( 182 ). In conjunction with assessment of end-tidal carbon monoxide, corrected for ambient CO, the task of early detection of the at-risk neonate is somewhat easier ( 183 ). Increased vigilance has been emphasized in recent years, because of the re-emergence of kernicterus, a disorder that should be entirely preventable ( 184 ).
Phototherapy.
Phototherapy started in 1958 ( 185 ), and 10 y after its introduction, it was reintroduced in the United States for use in preterm infants by Jerold Lucey ( 186 ). There was contentious debate about its safety in the early days, but it now is an established treatment modality that helps to prevent kernicterus in preterm infants. Although overhead lights are usually used, the application of eye shades often upsets parents. Bili-blankets, using fiberoptics, now can be wrapped around the infant to avoid the need for eye protection ( 187 ); also a new phototherapy light source, using high-intensity, light-emitting diodes, has been shown to be at least as effective as conventional phototherapy ( 188 ).
CHANGES IN ROUTINE PRACTICES
Routine neonatal care has changed substantially in the past 50 y. Although well-infant nurseries are primarily the domain of the general pediatrician, they provide the basis of neonatology. Since 1983, the AAP has published jointly with the American College of Obstetricians and Gynecologists “Guidelines for Perinatal Care,” now in its fifth edition ( 189 ).
Previously, it was thought necessary to wear caps, gowns, and masks before entering any nursery. Today the emphasis is on hand washing. Other areas of care that have changed include umbilical cord care, eye prophylaxis, sleep position ( 105 ), and identification procedures. Three additions to routine care are 1 ) administration of Hepatitis B vaccine, 2 ) hearing screening ( 190 ), and 3 ) use of car seats ( 191 ). With regard to this last item, attitudes toward automobile safety have changed for all ages, but special considerations apply to premature infants at discharge ( 192 ).

ROLE OF WOMEN IN NEONATOLOGY
Perhaps in no other pediatric specialty has the role of women been greater than in neonatology. As already mentioned, Virginia Apgar and Mary-Ellen Avery contributed seminal works that had an enormous influence on the care of neonates. Other pioneers were Mary Crosse at the Sorrento Maternity Hospital in Birmingham, England; Beryl Corner in Bristol, England; Ethel Dunham of Yale University, New Haven, Connecticut; Lula Lubchenco in Denver, Colorado; Murdina Desmond in Houston, Texas; Joan Hodgman in Los Angeles; and Mildred Stahlman in Nashville, Tennessee.
Maria Delivoria-Papadopoulos was one of the first to investigate the use of positive-pressure ventilation in RDS, and Mildred Stahlman was using the negative-pressure ventilator at approximately the same time. The perinatal pathologists Edith Potter, in Chicago, and Jeanne-Claudie Larroche, in Paris, and the neuropathologist Betty Banker, in Cleveland, have greatly influenced our understanding of neonatal disorders.
Claudine Amiel-Tison, in Paris, learned neonatal neurologic assessment at the feet of André Thomas and Suzanne St. Anne-Dargassies and then expanded their observations to the very preterm infant, with extended follow-up observations. Other women who have made careful follow-up of preterm infants their life's work are Cecil Mary Drillien in Edinburgh; Lula Lubchenco in Denver; Ann Stewart in London; Betty Vohr in Providence, Rhode Island; Maureen Hack in Cleveland; and Saroj Saigal in Hamilton, Ontario, at McMaster University. For many years, the name of Lily Dubowitz was synonymous with gestational age assessment, and her scoring system was more recently modified by Jean Ballard.
Bilirubin metabolism and the prevention of kernicterus have been the domain of Audrey Brown, a neonatal hematologist, and Lois Johnson, whereas the prevention of ROP (retrolental fibroplasia) has greatly benefited from the work of Kate Campbell, in Australia, in the early years (1951) [see ( 18 )] and of Dale Phelps in more recent years. Roberta Ballard was long an advocate of antenatal steroids to prevent RDS; Billie Short was a leader in the use of ECMO; and the first reported use of computed tomography to detect IVH was by Lu-Ann Papile and co-workers.
Renate Huch, with her obstetrician husband Albert, in Germany and later in Switzerland, introduced both transcutaneous Po 2 and Pco 2 electrodes into perinatal medicine, especially into neonatology. The team of Victoria Smallpeice and Pamela Davies in Oxford, England, introduced early feeding of human breast milk to premature infants at a time when it was considered controversial.
Leaders (chairpersons) of the Perinatal Section of the AAP include Marilyn Escobedo and Ann Stark. Lillian Blackmon recently chaired the Committee on Fetus and Newborn of the AAP, and Sherin Devaskar recently became the Editor-in Chief of Pediatric Research .
NEONATOLOGY AND THE PEDIATRIC SOCIETIES
The American Pediatric Society was established in 1888, and in its centennial year, the newborn infant was one of four featured areas at the annual meeting ( 193 ). In the late 1960s and 1970s, the American Pediatric Society–Society for Pediatric Research meeting was a very exciting place to be, with many new discoveries presented. In the words of Murdina Desmond: “As the end of the baby boom (1946–64) approached, neonatology took its place as a major division of pediatrics. At pediatrics meetings, newborn research was presented to standing-room only audiences” ( 6 ).
The Perinatal Section was established within the AAP in late 1974. A list of chairpersons is provided in Table 1 . The first 25 y of the Perinatal Section were summarized by L. Joseph Butterfield, an early promoter and staunch supporter of the section until his death in 1999 ( 194 ).
In 1975, both the first examination of the Sub-Board of Neonatal-Perinatal Medicine (for the chairpersons, see Table 2 ) and the first meeting of the Perinatal Section were held. The first chairman of the section, William H. Tooley, presided over the presentation of the Apgar Award to Clement A. Smith, who had recently (in 1974) relinquished the Editorship of Pediatrics to Jerold F. Lucey (another neonatologist, who still holds that position!). The list of recipients of the Apgar Award, presented to “an individual whose career has had a continuing influence on the well-being of newborn infants,” is provided in Table 3 .
In 1993, Dr. Leonore Ballowitz from Berlin, Germany, gave the first Thomas E. Cone, Jr., M.D., Lecture on Perinatal History on “The Life of Arvo Ylppö. Other speakers and topics are provided in Table 4 .
ERRORS IN NEONATOLOGY
Although it would be comforting to think that modern neonatal care evolved seamlessly in a series of logical steps, there were many missteps along the way. Many of these were outlined by Dr. William Silverman in his book Retrolental Fibroplasia ( 18 ). In addition, Dr. Alex Robertson recently published three papers detailing some major mistakes that have occurred in neonatology ( 195 – 197 ). The second and third papers are concerned with errors that occurred since 1950. A summation is provided in Table 5 .
One example is the uncontrolled introduction of gastrostomy feeding for preterm infants in the early 1960s. Gastrostomy feeding was initially introduced as an adjunct to care of infants on ventilators. Unfortunately, this technique was rapidly introduced to the care of other preterm infants. It was abandoned at the end of the 1960s, when a randomized, controlled trial showed no benefit of gastrostomy; in fact, both mortality and morbidity were increased in the gastrostomy group ( 198 ).
WHAT DOES THE FUTURE HOLD?
In some ways, the future is now. Certain activities are occurring in some centers that are likely to be reproduced and become standard operating procedure in the future. The difficulty is to know which of these activities will persist. Ten years ago, I would have strongly predicted that liquid ventilation with perfluorocarbons ( 199 ) would be a major therapeutic technique in most university or research centers at the present time. However, it has not emerged as the force that many would have predicted.
Simulation techniques.
My colleague Lou Halamek has developed a simulated delivery room in which realistic maternal and infant mannequins can have their vital signs manipulated so that students, residents, nurses, etc. can practice resuscitation and other emergency responses while being videotaped. Such simulation activity allows people to become proficient and respond appropriately, before being subjected to a real, live, situation. There is also simulation for responding to emergencies in an infant who is on ECMO ( http://cape.lpch.org ). Other centers are likely to adopt these approaches, and in the future, they may evolve into the use of virtual reality techniques.
Multidisciplinary/multicenter studies.
In recent years, the National Institute on Child Health and Human Development network and the Vermont-Oxford Network (VON) have attempted to answer questions about clinical medicine by developing research protocols that can be used in a large number of centers to answer those questions in a much shorter time frame than would have been possible several years ago.
A number of quality improvement initiatives have also been started. A select group of ∼ 10 centers in the VON engaged in quality improvement cycles focusing on specific goals during the 1990s ( 200 ). By gathering data from a large number of centers, benchmark centers, with the lowest rates, could be identified and used to evaluate how improvements could be made in other centers. The VON database, now used by >400 NICUs around the world, is limited to neonates with birth weights <1500 g (VLBW).
A more recent VON quality improvement initiative was titled NIC/Q 2000 and involved a larger number of centers ( 201 ). In California, there was the development of the California Perinatal Quality Care Collaborative, which includes larger infants who are sick, as well as the VLBW infants. Individual centers can evaluate themselves against the total cohort to understand where possible improvements might occur. This allows interventions to be introduced and cycles of change to occur ( 202 ).
It seems likely that these initiatives will expand even further in the future and will allow the best practices to be identified and endorsed. I believe that such evidence-based medicine will be very important in the future ( 203 ).
Gene therapy.
I would have predicted a decade ago that gene therapy would be a daily occurrence in the year 2004, but there have been a number of setbacks in translating potential into practice. Nevertheless, it seems likely that the difficulties associated with this technique will be overcome in the future. After identifying a single genetic defect as the cause of a problem in a neonate, it will be possible to replace the defective gene.
Biologic reporters.
Light-emitting enzymes, luciferases, can label genes and cells. These internal light sources can be detected externally and become biologic indicators or reporters. This bioluminescent imaging has been used with target and therapeutic genes in living laboratory animals to analyze gene delivery and to monitor gene expression and immune therapies ( 204 , 205 ). Extension of these techniques to human newborn infants seems likely.
Artificial placenta.
One of the topics that has been mentioned for as long as I have been in neonatology is the development of an artificial placenta. This futuristic idea remains just that, and I doubt that hooking up an infant to an artificial placenta will occur any time soon.
The changing role of pediatricians.
With every passing year, the pediatric residency program seems to add another requirement that residents must satisfy to be a certified pediatrician. Over the past decade or more, the amount of time that residents spend in critical care units has been cut back substantially. Consequently, today's pediatric residents are not as well prepared to deal with unusual findings or neonatal emergencies. This has resulted in increasing reliance on neonatologists to provide consultations in well-infant nurseries, with further erosion of the general pediatrician's skills with the neonate. This trend is likely to continue and may result in the expansion of the neonatal nurse practitioner workforce ( 206 ).
Rapid identification of infectious disease.
Presently, a number of viruses and bacteria can be detected using PCR techniques to make a rapid diagnosis ( 130 ). In the future, it seems likely that specimens will be screened with PCR to detect a battery of pathogenic organisms, and more judicious use of antibiotics will be needed to prevent the emergence of resistant organisms.
New vaccines.
Considerable progress has been made in developing GBS vaccines to individual subtypes ( 207 ). In the future, a polyvalent vaccine should be available as will a vaccine against HIV.
Blood substitutes.
Although each transfusion of blood in the neonate is comparatively small, NICUs are among the heaviest users of blood banks. Despite all of the safeguards to keep the blood supply free from contamination, it still seems worthwhile to seek blood substitutes.
Technological advances.
It is already possible to gain access to fetal cells and to grow them selectively. This offers therapeutic potential ( 208 ), and such tissue engineering has already occurred ( 209 ).
There has been a recent surge of interest in harvesting blood from the placenta to provide autologous blood transfusion in preterm infants ( 210 ), which minimizes demands for donor blood and its attendant risks.
Newer imaging techniques have been described in animal or experimental models ( 152 ). These are likely to reach the bedside soon along with skin surface electrodes that can measure glucose, electrolytes, and other biochemical determinations.
Better understanding.
There are many areas that still require elucidation and may yield answers with practical applications. Examples are 1 ) a better understanding of thrombophilia, which on the maternal side may be implicated in preeclampsia and IUGR and in the neonate may influence thrombotic complications; 2 ) a better understanding of organ damage and repair; and 3 ) an improved recognition and treatment of genetic disorders. Perhaps most important of all would be a better understanding of how to prevent prematurity.
In the past 50 y, there has been remarkable change in the care of the neonate. Particularly, the outlook for infants with birth weights of ∼ 1 kg has changed from 95% mortality to 95% survival. This report provides an overview of the development of the most important principles and concepts that form the basis of neonatology today. As neonatologists, we owe an enormous debt to our predecessors, but we are still faced with many challenges for the future.
Abbreviations
American Academy of Pediatrics
bronchopulmonary dysplasia
continuous positive airway pressure
extracorporeal membrane oxygenation
extremely low birth weight
hyaline membrane disease
inhaled nitric oxide
intrauterine growth restriction
intraventricular hemorrhage
ratio of lecithin, phosphatidyl choline, to sphingomyelin
neonatal intensive care unit
patent ductus arteriosus
positive end expiratory pressure
persistent pulmonary hypertension of the newborn
periventricular hemorrhage
respiratory distress syndrome
retinopathy of prematurity
sudden infant death syndrome
very low birth weight
Vermont-Oxford Network
Readers may wish to consult a series of “Historical Perspectives” that have appeared monthly since October 2002 in the journal “NeoReviews,” available at www.NeoReviews.org , published by the AAP. In addition, the development of neonatal intensive care in the United Kingdom has been chronicled in “Origins of Neonatal Intensive Care in the UK.” In: Christie DA, Tansey EM (eds) Wellcome Witnesses to Twentieth Century Medicine, Vol 9 Wellcome Trust, London, 2001, pp 1–75.
Ballantyne JW 1923 The new midwifery. BMJ 1 : 617–621
CAS PubMed PubMed Central Google Scholar
Grulee CG 1939 The newborn. President's address to the American Pediatric Society. Am J Dis Child 58 : 1–7
Google Scholar
Apgar V 1953 A proposal for a new method of evaluation of the newborn infant. Curr Res Anesth Analg 32 : 260–267
Article CAS PubMed Google Scholar
Cone TE Jr 1985 History of the Care and Feeding of the Premature Infant. Little Brown Co, Boston
Cone TE Jr 1983 Perspectives in Neonatology. In: Smith GF, Vidyasagar D (eds) Historical Review and Recent Advances in Neonatal and Perinatal Medicine , Vol 1 . Mead Johnson Nutritional Division, pp 9–33
Desmond MM 1998 Newborn Medicine and Society. European Background and American Practice (1750–1975) . Eakin Press, Austin
Hess JH, Lundeen EC 1941 The Premature Infant. Its Medical and Nursing Care . J.B. Lippincott, Philadelphia
Parmalee AH Sr 1952 Management of the Newborn . Year Book Publishers, Chicago
Dunham EC 1948 Premature Infants. A Manual for Physicians . Children's Bureau Publication No. 325, U.S. Government Printing Office, Washington, DC
Crosse VM 1945 The Premature Baby . Churchill Livingstone, London
Barcroft J 1946 Researches on Prenatal Life , Vol 1 . Blackwell Scientific Publications, Oxford
Smith CA 1947 The Physiology of the Newborn Infant . C.C. Thomas, Springfield
Dawes GS 1968 Foetal and Neonatal Physiology . Year Book Medical Publishers, Chicago
Schaffer AJ 1960 Diseases of the Newborn . WB Saunders, Philadelphia
O'Brien D, Ibbot FA 1964 Laboratory Manual of Pediatrics. Micro and Ultramicro-biochemical Techniques , 3rd Ed. Harper and Ross
Nelson NM 1983 Perinatal medicine. In: Smith GF, Vidyasagar D (eds) Historical Review and Recent Advances in Neonatal and Perinatal Medicine , Vol 1 . Mead Johnson Nutritional Division, pp 3–8
Silverman WA 1979 Incubator-baby side shows (Dr. Martin A. Couney). Pediatrics 64 : 127–141
CAS PubMed Google Scholar
Silverman WA 1980 Retrolental Fibroplasia. A Modern Parable . Grune and Stratton, New York
Baker JP 2000 The incubator and the medical discovery of the premature infant. J Perinatol 20 : 321–328
Cone TE Jr 1981 The first published report of an incubator for use in the care of the premature infant (1857). Am J Dis Child 135 : 658–660
PubMed Google Scholar
Silverman WA, Fertig JW, Berger AP 1958 The influence of the thermal environment upon the survival of newly born premature infants. Pediatrics 22 : 876–886
Sinclair JC 1970 Heat production and thermoregulation in the small-for date infant. Pediatr Clin North Am 17 : 147–158
Hull D 1966 The structure and function of brown adipose tissue. Br Med Bull 22 : 92–96
1970 Symposium on “the small-for-date infant.”. Pediatr Clin North Am 17 : 1–202
Hey EL 1975 Thermal neutrality. Br Med Bull 31 : 69–74
Dafoe AR 1940 The survival of the Dionne quintuplets. Am J Obstet Gyncol 39 : 159–164
Schreiner RL, Brady MS, Erust JA, Lemons JA 1982 Lack of lactobezoars in infants given predominantly whey protein formulas. Am J Dis Child 136 : 437–439
Zimmerman DR, Guttman N 2001 “Breast is best”: knowledge among low-income mothers is not enough. J Hum Lact 17 : 14–19
Lucas A, Hudson GJ 1984 Preterm milk as a source of protein for low birthweight infants. Arch Dis Child 59 : 831–836
Toubas PL 2001 Unanticipated consequences of early advances in newborn medicine. Perinatal Section News 27 : 1–4
Royce S, Tepper C, Watson W, Day R 1951 Indwelling polyethylene nasogastric tube for feeding premature infants. Pediatrics 8 : 79–81
Smallpeice V, Davies PA 1964 Immediate feeding of premature infants with undiluted breast-milk. Lancet 13 : 1349–1352
Cornblath M, Wybregt SH, Baens GS, Klein R 1964 Symptomatic neonatal hypoglycemia. Studies of carbohydrate metabolism in the newborn infant, VIII. Pediatrics 33 : 388–402
Dudrick SJ, Wilmore DW, Vars HM, Rhoads JE 1968 Long-term total parenteral nutrition with growth, development, and positive nitrogen balance. Surgery 64 : 134–142
Dudrick SJ, Rhoads JE 1972 Total intravenous feeding. Sci Am 226 : 73–80
Driscoll JM Jr, Heird WC, Schullinger JN, Gongaware RD, Winters RW 1972 Total intravenous alimentation in low-birth-weight infants: a preliminary report. J Pediatr 81 : 145–153
Shaw JCL 1973 Parenteral nutrition in the management of sick low birthweight infants. Pediatr Clin North Am 20 : 333–358
Heird WC, Kashyap S, Gomez MR 1991 Parenteral alimentation of the neonate. Semin Perinatol 15 : 493–502
Kerner JA Jr, Sunshine P 1979 Parenteral alimentation. Semin Perinatol 3 : 417–434
Denne SC 2001 Protein and energy requirements in preterm infants. Semin Neonatol 6 : 377–382
Putet G 2000 Lipid metabolism of the micropremie. Clin Perinatol 27 : 57–69
McClure RJ 2001 Trophic feeding of the preterm infant. Acta Paediatr Suppl 90 : 19–21
Lubchenco LO, Hansman C, Dressler M, Boyd E 1963 Intrauterine growth as estimated from liveborn birth-weight data at 24 to 42 weeks of gestation. Pediatrics 32 : 793–800
Usher RH, McLean F 1969 Intrauterine growth of live-born Caucasian infants at sea level: standards obtained from measurements in 7 dimensions of infants born between 25 and 44 weeks of gestation. J Pediatr 74 : 901–910
Battaglia FC, Lubchenco LO 1967 A practical classification of newborn infants by weight and gestational age. J Pediatr 71 : 159–163
Farquhar JW 1959 The child of the diabetic woman. Arch Dis Child 34 : 76–96
Gellis SS, Hsia DY 1959 The infant of diabetic mother. Am J Dis Child 97 : 1–41
CAS Google Scholar
Cornblath M 1961 Infants of diabetic mothers. Pediatrics 28 : 1024–1026
Gruenwald P 1963 Chronic fetal distress and placental insufficiency. Biol Neonate 33 : 215–265
Warkany J, Monroe BB, Sutherland BS 1961 Intrauterine growth retardation. Am J Dis Child 102 : 249–279
Barker DJ, Eriksson JG, Forsen T, Osmond C 2002 Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol 31 : 1235–1239
Clifford SH 1954 Post-maturity with placental dysfunction: clinical syndrome and pathologic findings. J Pediatr 44 : 1–13
Boylan P 1990 Induction of labor, complications of labor, and postmaturity. Curr Opin Obstet Gynecol 2 : 31–35
Lees MH 1970 Cyanosis of the newborn infant. Recognition and clinical evaluation. J Pediatr 77 : 484–498
Saugstad OD 2001 Update on oxygen radical disease in neonatology. Curr Opin Obstet Gynecol 13 : 147–153
Apgar V, Holaday DA, James LS, Weisbrot IM, Berrien C 1958 Evaluation of the newborn infant-Second report. JAMA 168 : 1985–1988
Casey BM, McIntire DD, Leveno KJ 2001 The continuing value of the Apgar score for the assessment of newborn infants. N Engl J Med 344 : 467–471
James LS 1975 Fond memories of Virginia Apgar. Pediatrics 55 : 1–4
Saugstad OD 2001 Resuscitation of newborn infants with room air or oxygen. Semin Neonatol 6 : 233–239
Hutchison JH, Kerr MM, Williams KG, Hopkinson WI 1963 Hyperbaric oxygen in the resuscitation of the newborn. Lancet 13 : 1019–1022
Gregory GA, Gooding CA, Phibbs RH, Tooley WH 1974 Meconium aspiration in infants—a prospective study. J Pediatr 85 : 848–852
Carson BS, Losey RW, Bowes WA Jr, Simmons MA 1976 Combined obstetric and pediatric approach to prevent meconium aspiration syndrome. Am J Obstet Gynecol 126 : 712–715
Keenan WJ 1998 The first decade: the neonatal resuscitation program. In: Fanaroff AA, Maisels MJ, Stevenson DK (eds) Year Book of Neonatal and Perinatal Medicine . Mosby, St. Louis, pp xxix–xxxi
Nelson KB 2003 Can we prevent cerebral palsy?. N Engl J Med 349 : 1765–1769
Brann AW Jr, Myers RE 1975 Central nervous system findings in the newborn monkey following severe in utero partial asphyxia. Neurology 25 : 327–338
Battin MR, Penrice J, Gunn TR, Gunn AJ 2003 Treatment of term infants with head cooling and mild systemic hypothermia (35.0°C and 34.5°C) after perinatal asphyxia. Pediatrics 111 : 244–251
Avery ME, Mead J 1959 Surface properties in relation to atelectasis and hyaline membrane disease. Am J Dis Child 97 : 517–523
Chu J, Clements JA, Cotton E, Klaus MH, Sweet AY, Thomas MA, Tooley WH 1965 The pulmonary hypoperfusion syndrome. Pediatrics 35 : 733–742
Usher RH 1963 Reduction of mortality from respiratory distress syndrome of prematurity with early administration of intravenous glucose and sodium bicarbonate. Pediatrics 32 : 966–975
Swyer PR 1970 Symposium on artificial ventilation. Summary of conference proceedings. Biol Neonate 16 : 191–195
Northway WH Jr, Rosan RC, Porter DY 1967 Pulmonary disease following respirator therapy of hyaline membrane disease. Bronchopulmonary dysplasia. N Engl J Med 276 : 357–368
Philip AG 1975 Oxygen plus pressure plus time: the etiology of bronchopulmonary dysplasia. Pediatrics 55 : 44–50
Bancalari E, Claure N, Sosenko IR 2003 Bronchopulmonary dysplasia: changes in pathogenesis, epidemiology and definition. Semin Neonatol 8 : 63–71
Kuhns LR, Bednarek FJ, Wyman ML, Roloff DW, Borer RC 1975 Diagnosis of pneumothorax and pneumomediastinum in the neonate by transillumination. Pediatrics 56 : 355–360
Donn SM, Kuhns LR 1983 Pediatric Transillumination . Year Book Medical Publishers, Chicago
Soll RF, Blanco F 2001 Natural surfactant extract versus synthetic surfactant for neonatal respiratory distress. Cochrane Database Syst Rev ( 2): CD000144
Nelle M, Zilow EP, Linderkamp O 1997 Effects of high-frequency oscillatory ventilation on circulation in neonates with pulmonary interstitial emphysema or RDS. Intensive Care Med 23 : 671–676
Gregory GA, Kitterman JA, Phibbs RH, Tooley WH, Hamilton WK 1971 Treatment of idiopathic respiratory-distress syndrome with continuous positive airway pressure. N Engl J Med 284 : 1333–1340
Kattwinkel J, Fleming D, Cha CC, Fanaroff AA, Klaus MH 1973 A device for administration of continuous positive airway pressure by the nasal route. Pediatrics 52 : 131–134
Gluck L, Kulovich MV 1973 Fetal lung development. Current concepts. Pediatr Clin North Am 20 : 367–379
Adams FH, Desilets DT, Towers B 1967 Control of flow of fetal lung fluid at the laryngeal outlet. Respir Physiol 2 : 302–309
Piper JM, Langer O 1993 Does maternal diabetes delay fetal pulmonary maturity?. Am J Obstet Gynecol 168 : 783–786
Piper JM 2002 Lung maturation in diabetes in pregnancy: if and when to test. Semin Perinatol 26 : 206–209
Liggins GC, Howie RN 1972 A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics 50 : 515–525
Collaborative Group on Antenatal Steroid Therapy 1981 Effects of antenatal dexamethasone administration on the prevention of respiratory distress syndrome. Am J Obstet Gynecol 141 : 276–286
McCarthy M 1994 US recommendations for antenatal corticosteroids. Lancet 343 : 726
National Institute of Child Health and Human Development 1994 Report of the Consensus Development Conference on the Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes. U.S. Department of Health and Human Services , Washington, DC
Ment LR, Oh W, Ehrenkranz RA, Philip AG, Duncan CC, Makuch RW 1995 Antenatal steroids, delivery mode, and intraventricular hemorrhage in preterm infants. Am J Obstet Gynecol 172 : 795–800
Pattle RE 1955 Properties, function and origin of the alveolar lining layer. Nature 175 : 1125–1126
Clements JA 1957 Surface tension of lung extracts. Proc Soc Exp Biol Med 95 : 170–172
Fujiwara T, Maeta H, Chida S, Morita T, Watabe Y, Abe T 1980 Artificial surfactant therapy in hyaline-membrane disease. Lancet 1 : 55–59
Merritt TA, Hallman M, Bloom BT, Berry C, Benirschke K, Sahn D, Key T, Edwards D, Jarvenpaa AL, Pohjavuori M et al. 1986 Prophylactic treatment of very premature infants with human surfactant. N Engl J Med 315 : 785–790
Corbet AJ, Long WA, Murphy DJ, Garcia-Prats JA, Lombardy LR, Wold DE 1991 Reduced mortality in small premature infants treated at birth with a single dose of a synthetic surfactant. J Paediatr Child Health 27 : 245–249
Morley CJ 1989 Prophylactic treatment of premature babies with artificial surfactant (ALEC). Dev Pharmacol Ther 13 : 182–183
Speer CP, Robertson B, Curstedt T, Halliday HL, Compagnone D, Gefeller O, Harms K, Herting E, McClure G, Reid M et al. 1992 Randomized European multicenter trial of surfactant replacement therapy for severe neonatal respiratory distress syndrome: single versus multiple doses of Curosurf. Pediatrics 89 : 13–20
Whitsett JA, Weaver TE 2002 Hydrophobic surfactant proteins in lung function and disease. N Engl J Med 347 : 2141–2148
Horbar JD, Wright EC, Onstad L 1993 The Members of the National Institute of Child Health and Human Development Neonatal Research Network. Decreasing mortality associated with the introduction of surfactant therapy: an observational study of neonates weighing 601 to 1300 grams at birth. Pediatrics 92 : 191–196
Daily WJ, Klaus M, Meyer HB 1969 Apnea in premature infants: monitoring, incidence, heart rate changes, and an effect of environmental temperature. Pediatrics 43 : 510–518
Sinclair JC 1970 The premature baby who “forgets to breathe.”. N Engl J Med 282 : 508–509
Huch R, Huch A, Lübbers DW 1973 Transcutaneous measurement of blood Po2 (tcPo2)—method and application in perinatal medicine. J Perinat Med 1 : 183–191
Hay WW Jr, Thilo E, Curlander JB 1991 Pulse oximetry in neonatal medicine. Clin Perinatol 18 : 441–472
Kuzemko JA, Paala J 1973 Apnoeic attacks in the newborn treated with aminophylline. Arch Dis Child 48 : 404–406
Aranda JV, Gorman W, Bergsteinsson H, Gunn T 1977 Efficacy of caffeine in treatment of apnea in the low-birth-weight infant. J Pediatr 90 : 467–472
Firstman R, Talan J 1997 The Death of Innocents: A True Story of Murder, Medicine, and High-Stake Science. Bantam Books, New York
American Academy of Pediatrics 1992 AAP Task Force on Infant Positioning and SIDS: positioning and SIDS. Pediatrics 89 : 1120–1126
Friedman WF, Hirschklau MJ, Printz MP, Pitlick PT, Kirkpatrick SE 1976 Pharmacologic closure of patent ductus arteriosus in the premature infant. N Engl J Med 295 : 530–533
Heymann MA, Rudolph AM, Silverman NH 1976 Closure of the ductus arteriosus in premature infants by inhibition of prostaglandin synthesis. N Engl J Med 295 : 530–533
Elliott RB, Starling MB, Neutze JM 1975 Medical manipulation of the ductus arteriosus. Lancet 1 : 140–142
Kitterman JA, Phibbs RH, Tooley WH 1969 Aortic blood pressure in normal newborn infants during the first 12 hours of life. Pediatrics 44 : 959–968
So KW, Fok TF, Ng PC, Wong WW, Cheung KL 1997 Randomised controlled trial of colloid or crystalloid in hypotensive preterm infants. Arch Dis Child Fetal Neonatal Ed 76 : F43–F46
Roze JC, Tohier C, Maingueneau C, Lefevre M, Mouzard A 1993 Response to dobutamine and dopamine in the hypotensive very preterm infant. Arch Dis Child 69 : 59–63
Gersony WM 1973 Persistence of the fetal circulation: a commentary. J Pediatr 82 : 1103–1106
Goetzman BW, Sunshine P, Johnson JD, Wennberg RP, Hackel A, Merten DF, Bartoletti AL, Silverman NH 1976 Neonatal hypoxia and pulmonary vasospasm: response to tolazoline. J Pediatr 89 : 617–621
Schreiber MD, Heymann MA, Soifer SJ 1986 Increased arterial pH, not decreased PaCO2, attenuates hypoxia-induced pulmonary vasoconstriction in newborn lambs. Pediatr Res 20 : 113–117
Tolsa JF, Cotting J, Sekarski N, Payot M, Micheli JL, Calame A 1995 Magnesium sulphate as an alternative and safe treatment for severe persistent pulmonary hypertension of the newborn. Arch Dis Child Fetal Neonatal Ed 72 : F184–F187
Murphy JD, Rabinovitch M, Goldstein JD, Reid LM 1981 The structural basis of persistent pulmonary hypertension of the newborn infant. J Pediatr 98 : 968–967
Kinsella JP, Neish Sr, Ivy DD, Shaffer E, Abman SH 1993 Clinical responses to prolonged treatment of persistent pulmonary hypertension of the newborn with low doses of inhaled nitric oxide. J Pediatr 123 : 103–108
Roberts JD, Polaner DM, Lang P, Zapol WM 1992 Inhaled nitric oxide in persistent pulmonary hypertension of the newborn. Lancet 340 : 818–819
Bartlett RH, Andrews AF, Toomasian JM, Haiduc NJ, Gazzaniga AB 1982 Extracorporeal membrane oxygenation for newborn respiratory failure: forty-five cases. Surgery 92 : 425–433
O'Rourke PP, Crone RK, Vacanti JP, Ware JH, Lillehei CW, Parad RB, Epstein MF 1989 Extracorporeal membrane oxygenation and conventional medical therapy in neonates with persistent pulmonary hypertension of the newborn: a prospective randomized study. Pediatrics 84 : 957–963
UK Collaborative ECMO Trail Group (Field DJ) 1996 UK collaborative randomized trial of neonatal extracorporeal membrane oxygenation. Lancet 348 : 75–82
Winsberg F 1972 Echocardiography of the fetal and newborn heart. Invest Radiol 7 : 152–158
Eichenwald H, Kotsevalov O, Fasso LA 1960 The “cloud baby”: an example of bacterial-viral interaction. Am J Dis Child 100 : 161–173
Yow EM, Yow MD, Womack GK, Sakuma T, Monzon OT 1958 The management of hospital-acquired staphylococcal infections. Am Arch Intern Med 102 : 948–959
Lockhart JD 1972 How toxic is Hexachlorophene?. Pediatrics 50 : 229–235
Philip AG, Hewitt JR 1980 Early diagnosis of neonatal sepsis. Pediatrics 65 : 1036–1041
Sabel KG, Hanson LA 1974 The clinical usefulness of C-reactive protein (CRP) determinations in bacterial meningitis and septicemia in infancy. Acta Paediatr Scand 63 : 381–388
Manroe BL, Rosenfeld CR, Weinberg AG, Browne R 1977 The differential leukocyte count in the assessment and outcome of early-onset neonatal group B streptococcal disease. J Pediatr 91 : 632–637
Manroe BL, Weinberg AG, Rosenfeld CR, Browne R 1979 The neonatal blood count in health and disease. I. Reference values for neutrophilic cells. J Pediatr 95 : 89–98
Nissen MD, Sloots TP 2002 Rapid diagnosis in pediatric infectious diseases: the past, the present and the future. Pediatr Infect Dis J 21 : 605–612
McCracken GH Jr 1973 Group B streptococci: the new challenge in neonatal infections. J Pediatr 82 : 703–706
Baker CJ 1979 Group B streptococcal infections in neonates. Pediatr Rev 1 : 5–1
Benitz WE 2002 Perinatal treatment to prevent early onset group B streptococcal sepsis. Semin Neonatol 7 : 301–314
McCracken GH Jr, Mize SG, Threlkeld N 1980 Intraventricular gentamicin therapy in gram-negative bacillary meningitis of infancy. Report of the Second Neonatal Meningitis Cooperative Study Group. Lancet 1 : 787–791
Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, Lemons JA, Donovan EF, Stark AR, Tyson JE, Oh W, Bauer CR, Korones SB, Shankaran S, Laptook AR, Stevenson DK, Papile LA, Poole WK 2002 Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics 110 : 285–291
Chapman RL 2003 Candida infections in the neonate. Curr Opin Pediatr 15 : 97–102
Giles JP, Cooper LZ, Krugman S 1965 The rubella syndrome. J Pediatr 66 : 434–437
Nahmias AJ 1974 The TORCH complex. Hosp Pract 9 : 65–73
Leland D, French ML, Kleiman MB, Schreiner RL 1983 The use of TORCH titers. Pediatrics 72 : 41–43
Kumar ML 1991 Human parvovirus B19 and its associated diseases. Clin Perinatol 18 : 209–225
Beckerman KP 2002 Mothers, orphans, and prevention of paediatric AIDS. Lancet 359 : 1168–1169
Stevenson JK, Oliver TK Jr, Graham CB, Bell RS, Gould VE 1971 Aggressive treatment of neonatal necrotizing enterocolitis: 38 patients with 25 survivors. J Pediatr Surg 6 : 28–35
Bisquera JA, Cooper TR, Berseth CL 2002 Impact of necrotizing enterocolitis on length of stay and hospital charges in very low birth weight infants. Pediatrics 109 : 423–428
Papile LA, Burstein J, Burstein R, Koffler H 1978 Incidence and evolution of sub-ependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1500 gm. J Pediatr 92 : 529–534
Pape KE, Blackwell RJ, Cusick G, Sherwood A, Houang MT, Thorburn RJ, Reynolds EO 1979 Ultrasound detection of brain damage in preterm infants. Lancet 1 : 1261–1264
Lazzara A, Ahmann P, Dykes F, Brann AW Jr, Schwartz J 1980 Clinical predictability of intraventricular hemorrhage in preterm infants. Pediatrics 65 : 30–34
Philip AG, Allan WC, Tito AM, Wheeler LR 1989 Intraventricular hemorrhage in preterm infants: declining incidence in the 1980s. Pediatrics 84 : 797–801
Ment LR, Oh W, Ehrenkranz RA, Philip AG, Vohr B, Allan W, Duncan CC, Scott DT, Taylor KJ, Katz KH et al. 1994 Low-dose indomethacin and prevention of intraventricular hemorrhage: a multicenter randomized trial. Pediatrics 93 : 543–550
Horbar JD, Badger GJ, Carpenter JH, Fanaroff AA, Kilpatrick S, LaCorte M, Phibbs R, Soll RF, Members of the Vermont Oxford Network 2002 Trends in mortality and morbidity for very low birth weight infants, 1991–1999. Pediatrics 110 : 143–151
Lemons JA, Bauer CR, Oh W, Korones SB, Papile LA, Stoll BJ, Verter J, Temprosa M, Wright LL, Ehrenkranz RA, Fanaroff AA, Stark A, Carlo W, Tyson JE, Donovan EF, Shankaran S, Stevenson DK 2001 Very low birth weight outcomes of the National Institute of Child health and human development neonatal research network, January 1995 through December 1996. NICHD Neonatal Research Network. Pediatrics 107 : E1
Rhine WD, Ashwal S, Holshouser B, D'Arceuil HE, Van Houten JP, Contag CH, Benaron DA, Stevenson DK 1997 Non-invasive monitoring: the next generation. In: Fanaroff AA, Maisels MJ, Stevenson DK (eds) Yearbook of Neonatal and Perinatal Medicine, . Mosby, St. Louis, pp xvii–xlvii
Smith LE 2002 Pathogenesis of retinopathy of prematurity. Acta Paediatr Suppl 91 : 26–28
Multicenter trial of cryotherapy for retinopathy of prematurity: preliminary results 1988 Cryotherapy for Retinopathy of Prematurity Cooperative Group. Preliminary results. Pediatrics 88 : 697–706
Behrman RE, Babson GS, Lessel R 1971 Fetal and neonatal mortality in white middle class infants: mortality risks by gestational age and weight. Am J Dis Child 121 : 486–489
Drillien CM 1967 The long-term prospects for babies of low birth weight. Br J Hosp Med 1 : 937–944
Templeton A, Morris JK 1998 Reducing the risk of multiple births by transfer of two embryos after in vitro fertilization. N Engl J Med 339 : 573–577
Hansen M, Kurinczuk JJ, Bower C, Webb S 2002 The risk of major birth defects after intracytoplasmic sperm injection or in vitro fertilization. N Engl J Med 346 : 725–730
Schieve LA, Meikle SF, Ferre C, Peterson HB, Jeng G, Wilcox LS 2002 Low and very low birthweight in infants conceived with use of assisted reproductive technology. N Engl J Med 346 : 731–737
Usher RH 1971 Clinical implications of perinatal mortality statistics. Clin Obstet Gynecol 14 : 885–925
Philip AG, Little GA, Polivy DR, Lucey JF 1981 Neonatal mortality risk for the eighties: the importance of birth weight/gestational groups. Pediatrics 68 : 122–130
Butterfield LJ 1983 The impact of regionalization on neonatal outcome. In: Smith GF, Vidyasagar D (eds) Historical Review and Recent Advances in Neonatal and Perinatal Medicine , Vol 2 . Mead Johnson Nutritional Division, Evansville, pp 107–124
Butterfield LJ 1993 Historical perspectives of neonatal transport. Pediatr Clin North Am 40 : 221–239
Lubchenco LO, Butterfield LJ, Delaney-Black V, Goldson E, Koops BL, Lazotte DC 1989 Outcome of very low birth weight infants: does antepartum versus neonatal referral have a better impact on mortality, morbidity or long-term outcome?. Am J Obstet Gynecol 160 : 539–545
DeVries RG 1979 Responding to consumer demand: a study of alternative birth centers. Hosp Prog 60 : 48–51, 68
Frank JE, Rhodes TT, Edwards WH, Darnall RA, Smith BD, Little GA, Baker ER, Stys SJ, Flanagan VA 1999 The New Hampshire Perinatal Program: twenty years of perinatal outreach education. J Perinatol 19 : 3–8
Barnett ER, Leiderman PH, Grobstein R, Klaus M 1970 Neonatal separation: the maternal side of interactional deprivation. Pediatrics 45 : 197–205
Klaus MH, Kennell JH 1970 Mothers separated from their newborn infants. Pediatr Clin N Am 17 : 1015–1037
Klaus MH, Kennell JH 1982 Parent-Infant Bonding , 2nd Ed. C.V. Mosby, St. Louis
Brazelton TB 1973 Neonatal Behavioral Assessment Scale . JB Lippincott, Philadelphia
Halamek LP 2003 Prenatal consultation at the limits of viability. Neoreviews 4 : e153–e156
Meier PP, Brown LP 1996 State of the science. Breastfeeding for mothers and low birth weight infants. Nurs Clin North Am 31 : 351–365
Whitelaw A, Sleath K 1985 Myth of the marsupial mother: home care of very low birth weight babies in Bogota, Colombia. Lancet 1 : 1206–1208
Anderson GC 1991 Current knowledge about skin-to-skin (kangaroo) care for preterm infants. J Perinatol 11 : 216–226
Lesser AL 1963 Phenylketonuria and the Guthrie test. Pediatrics 32 : 940
Fisch RO, Anthony BF, Bauer H, Bruhl HH 1968 The effect of antibiotics on the results of the Guthrie test given to phenylketonuric patients. J Pediatr 73 : 685–689
Dussault JH, Coulombe P, Laberge C, Letarte J, Guyda H, Khoury K 1975 Preliminary report on a mass screening program for neonatal hypothyroidism. J Pediatr 86 : 670–674
Wilcken B, Wiley V, Hammond J, Carpenter K 2003 Screening newborns for inborn errors of metabolism by tandem mass spectrometry. N Engl J Med 348 : 2304–2312
Queenan JT 2002 Rh-disease: a perinatal success story. Obstet Gynecol 100 : 405–406
Kappas A, Drummond GS, Valaes T 2001 A single dose of Sn-mesoporphyrin prevents development of severe hyperbilirubinemia in glucose-6 phosphate dehydrogenase-deficient newborns. Pediatrics 108 : 25–30
Maisels MJ, Kring E 1998 Length of stay, jaundice and hospital readmission. Pediatrics 101 : 995–998
Yamanouchi I, Yamauchi Y, Igarashi I 1980 Transcutaneous bilirubinometry: preliminary studies of non-invasive transcutaneous bilirubin meter in Okayama National Hospital. Pediatrics 65 : 195–202
Bhutani VK, Johnson L, Sivieri EM 2001 Predictive ability of a predischarge hour-specific serum bilirubin for subsequent significant hyperbilirubinemia in healthy term and new-term newborns. Pediatrics 103 : 6–14
Stevenson DK, Fanaroff AA, Maisels MJ, Young BW, Wong RJ, Vreman HJ, MacMahon JR, Yeung CY, Seidman DS, Gale R, Oh W, Bhutani VK, Johnson LH, Kaplan M, Hammerman C, Nakamura H 2001 Prediction of hyperbilirubinemia in near-term and term infants. Pediatrics 108 : 31–39
Brown AK, Johnson L 1996 Loss of concern about jaundice and the re-emergence of kernicterus in full term infants in the era of managed care. In: Fanaroff AA, Klaus M (eds) Yearbook of Neonatal and Perinatal Medicine . Mosby-Year Book, St. Louis, pp xvii–xxviii
Dobbs RH, Cremer RJ 1975 Phototherapy. Arch Dis Child 50 : 833–836
Lucey J, Ferreiro M, Hewitt J 1968 Prevention of hyperbilirubinemia of prematurity by phototherapy. Pediatrics 41 : 1047–1054
Gale R, Dranitzki Z, Dollberg S, Stevenson DK 1990 A randomized controlled application of the Wallaby phototherapy system compared with standard phototherapy. J Perinatol 10 : 239–242
Seidman DS, Moise J, Ergaz Z, Laor A, Vreman HJ, Stevenson DK, Gale R 2000 A new blue light-emitting phototherapy device: a prospective randomized controlled study. J Pediatr 136 : 771–774
American Academy of Pediatrics; American College of Obstetricians and Gynecologists 2002 Care of the neonate. Guidelines for Perinatal Care , 5th Ed. American Academy of Pediatrics, Elk Grove Village
Erenberg A, Lemons J, Sia C, Trunkel D, Ziring P 1999 Newborn and infant hearing loss: detection and intervention. American Academy of Pediatrics. Task Force on Newborn and Infant Hearing, 1998–1999. Pediatrics 103 : 527–530
Colletti RB 1983 Hospital-based rental programs to increase car seat usage. Pediatrics 71 : 771–773
Bass JL, Mehta KA, Camara J 1993 Monitoring premature infants in car seats: implementing the American Academy of Pediatrics policy in a community hospital. Pediatrics 91 : 1137–1141
Pearson HA 1989 The Centennial History of the American Pediatric Society 1888–1988: A Century of Progress in Child Health. Yale University Print Service, New Haven, CT
Butterfield LJ 1999 The AAP Section on Perinatal Pediatrics 1974–1999. In: Fanaroff AA, Maisels MJ Stevenson DK (eds) Year Book of Neonatal and Perinatal Medicine . Mosby, St. Louis, pp xvii–xxiv
Robertson AF 2003 Reflections on errors in neonatology: I. The “hands-off” years, 1920 to 1950. J Perinatol 23 : 48–55
Robertson AF 2003 Reflections on errors in neonatology: II. The “heroic” years, 1950 to 1970. J Perinatol 23 : 154–161
Robertson AF 2003 Reflection on errors in neonatology: III. The “experienced years,” 1970 to 2000. J Perinatol 23 : 240–249
Vengusamy S, Pildes RS, Raffensperger JF, Levine HD, Cornbath M 1969 A controlled study of feeding gastrostomy in low birth weight infants. Pediatrics 43 : 815–820
Greenspan JS, Wolfson MR, Rubinstein SD, Shaffer TH 1990 Liquid ventilation of human preterm neonates. J Pediatr 117 : 106–111
Horbar JD, Rogowski J, Plsek PE, Delmore P, Edwards WH, Hocker J, Kantak AD, Lewallen P, Lewis W, Lewit E, McCarroll CJ, Mujsce D, Payne NR, Shiono P, Soll RF, Leahy K, Carpenter JH 2001 Collaborative quality improvement for neonatal intensive care. NIC/Q Project Investigators of the Vermont Oxford Network. Pediatrics 107 : 14–22
Horbar JD, Plsek PE, Leahy K 2003 NIC/Q 2000: establishing habits for improvement in neonatal intensive care units. Pediatrics 111 : e397–e410
Wirtschafter DD, Powers RJ 2004 Organizing regional perinatal quality improvement: global considerations and a case study of a local implementation. Neoreviews 5 : e50–e59
Soll RF, Andruscavage L 1999 The principles and practice of evidence-based neonatology. Pediatrics 103 : e215–e224
McCaffrey A, Kay MA, Contag CH 2003 Advancing molecular therapies through in vivo bioluminescent imaging. Mol Imaging 2 : 75–86
Contag CH, Stevenson DK 2001 In vivo patterns of heme oxygenase-1 transcription. J Perinatol 21 : S119–S124
Mitchell-DiCenso A, Guyatt G, Marrin M, Goeree R, Willan A, Southwell D, Hewson S, Paes B, Rosenbaum P, Hunsberger M, Baumann A 1996 A controlled trial of nurse practitioners in neonatal intensive care. Pediatrics 98 : 1143–1148
Paoletti LC, Madoff LC 2002 Vaccines to prevent neonatal GBS infection. Semin Neonatol 7 : 315–323
Kaviani A, Perry TE, Dzakovic A, Jennings RW, Ziegler MM, Fauza DO 2001 The amniotic fluid as a source of cells for fetal tissue engineering. J Pediatr Surg 36 : 1662–1665
Donati V, Arena S, Capilli G, Carrera G, Ciralli F, Liberatore A 2001 Reparation of a severe case of aplasia cutis congenita with engineered skin. Biol Neonate 80 : 273–276
Brune T, Garritsen H, Hentschel R, Louwen F, Harms E, Jorch G 2003 Efficacy, recovery, and safety of RBCs from autologous placental blood: clinical experience in 52 newborns. Transfusion 43 : 1210–1216
Download references
Acknowledgements
I thank Philip Sunshine, M.D., for encouragement, recollections, and suggestions.
Author information
Authors and affiliations.
Department of Pediatrics, Division of Neonatal and Developmental Medicine, Stanford University School of Medicine, Palo Alto, 94304, CA
Alistair G S Philip
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Alistair G S Philip .
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Philip, A. The Evolution of Neonatology. Pediatr Res 58 , 799–815 (2005). https://doi.org/10.1203/01.PDR.0000151693.46655.66
Download citation
Received : 23 July 2004
Accepted : 05 November 2004
Issue Date : 01 October 2005
DOI : https://doi.org/10.1203/01.PDR.0000151693.46655.66
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Impact of corticosteroid exposure on preterm labor in neonates eventually born at term.
- Céline Best
- Jean-Michel Hascoet
- Emeline Renard
Journal of Perinatology (2024)
Meeting the need for effective and standardized neonatology training: a pan-European Master’s Curriculum
- Deanna Santoro
- Devin A. Zibulsky
- Sven Wellmann
Pediatric Research (2024)
Buy-in and breakthroughs: the Overton window in neonatology for the resuscitation of extremely preterm infants
- Jeanne A. Krick
- Dalia M. Feltman
- Brian S. Carter
Journal of Perinatology (2023)
Ethical challenges in first-in-human trials of the artificial placenta and artificial womb: not all technologies are created equally, ethically
- Stephanie K. Kukora
- George B. Mychaliska
- Elliott Mark Weiss
Ophthalmologische Langzeitfolgen der Frühgeburtlichkeit – persistierend bis in das Erwachsenenalter
- Eva Mildenberger
- Alexander K. Schuster
Die Ophthalmologie (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List
History of Blood Pressure Measurement in Newborns and Infants
Kamil javorka.
- Author information
- Article notes
- Copyright and License information
Corresponding author: K. Javorka, Department of Physiology, Jessenius Faculty of Medicine, Comenius University, Malá Hora 4C, 036 01 Martin, Slovak Republic. E-mail: [email protected]
Corresponding author.
Received 2023 Jun 26; Accepted 2023 Jul 27; Collection date 2023 Oct.
The development of methods for measuring blood pressure (BP) in newborns and small children has a rich history. Methods for BP measuring in adults had to be adapted to this age group. For measuring BP in direct invasive way, a suitable approach had to be found to access the arterial circulation through the umbilical and later radialis artery. Currently, results obtained from direct invasive BP measurement are considered the “gold standard”. The development of non-invasive methods for BP measuring in newborns and children began with the use of von Basch's sphygmomanometer (1880). In 1899, Gustav Gärtner constructed the device, which was the basis for the flush method. After the discovery of the palpation and auscultation methods, these methods were also used for BP measurement in newborns and children, however, the BP values obtained in these ways were typically underestimated using excessively wide cuffs. From the auscultation method, methods utilizing ultrasound and infrasound to detect arterial wall movement and blood flow were later developed. The oscillometric method for BP measurement was introduced by E. J. Marey so early as in 1876. In 1912, P. Balard used the oscillometric technique to measure blood pressure in a large group of newborns. Through different types of oscillometers using various methods for detecting vascular oscillations (such as xylol method, impedance and volume plethysmography, etc.), the development has continued to assessment of vascular oscillations by modern sensor technology and software. For continuous non-invasive blood pressure measurement, the volume-clamp method, first described by Jan Peňáz in 1968, was developed. After modification for use in newborns, application of the cuff to the wrist instead of the finger, it is primarily used in clinical physiological studies to evaluate beat-to-beat BP and heart rate pressure variability, such as in the determination of the baroreflex sensitivity.
Keywords: History, Blood pressure measurement, Invasive, Non-invasive methods, Newborns, Oscillometry, Continuous blood pressure monitoring
Introduction
Monitoring blood pressure (BP) has become a routine practice in all age groups, including newborns. The development of BP measurement methods has progressed from invasive to non-invasive, from animal studies to human studies, and from adults to children and newborns.
The history of medicine lacks a comprehensive description of the development of blood pressure measurement methods in newborns and young children, in whom, for different reasons, conventional methods applicable to adult humans could not be applied. The aim of this paper is to present an overview of this history.
Development of methods for invasive blood pressure measurement
It is interesting that since the description of the blood circulation by W. Harvey (1628), more than 100 years have passed since blood pressure was first measured.
The first BP measurement was made by an invasive method in animals. Stephen Hales published the results of blood pressure measurements in animals in his work “Haemastatics” (1733), which appeared in the second volume of Statical Essays [ 1 ]. Up to more than 120 years has it been managed to measure BP in humans. Blood pressure in adult patients was measured invasively by the surgeon Faivre [ 2 ] in Lyon in 1856.
In 1929, Haselhorst [ 3 ] measured blood pressure in newborns by inserting a needle into the umbilical vein during a cesarean section in three term infants still in utero. The average pressure was 25 mm Hg. These experiments were mainly designed to determine the appropriate time to cut the umbilical cord and to ascertain the size of the placental transfusion.
In 1938, Woodbury et al . [ 4 ] inserted a needle into the umbilical artery shortly after birth in 37 newborns. Spontaneous closure of the umbilical vessels was delayed by immersing the body in warm water. They used a Hamilton’s manometer with pressure transmission to a silvered membrane, the deformation of which reflected light beams onto photographic film. In full-term newborns, they determined BP systolic as 80.1±8.1 mm Hg and diastolic BP 46.3±8.2 mm Hg. In preterm infants, the pressure was lower and correlated with gestational age. This study is considered groundbreaking, as previous attempts to measure blood pressure invasively were limited by the use of inappropriate manometers and should not be taken into consideration [ 5 ].
In the 1950s and 1960s, the development of suitable catheters, low dead-space transducers, sensors, particularly strain gauge type and electromanometers, took place. These technological developments enabled the further development of the method of direct measurement of neonatal blood pressure. Wallgren et al. [ 6 ] simultaneously recorded blood pressure using a catheter inserted into the umbilical artery together with intraesophageal pressure in the first few minutes after birth. Other authors, such as Rudolph et al. [ 7 ], began using invasive methods to measure BP in pathological newborns with respiratory distress syndrome (RDS).
In 1975, Todres et al. [ 8 ] tried percutaneous catheterization of the a. radialis in critically ill newborns. As today's practice has shown, their observation of the possibility of monitoring blood pressure also by this way was correct.
For ethical and practical reasons, non-invasive methods of blood pressure measurement began to be developed at the same time, used first in adults, later in children and newborns.
Development of methods for non-invasive blood pressure measurement
Mechanical sphygmomanometry.
The first non-invasive methods for the assessment of cardiovascular function were focused on the evaluation of the quantity and quality of arterial pulse with sphygmometers and sphygmographs.
Jules Herisson [ 9 ] constructed one such apparatus in 1833. His sphygmometer was composed of graduated glass tube terminating in a semi-globular steel ball closed by a membrane ( Fig. 1a ). The mercury reservoir was pressed manually against the wrist above the a. radialis until the oscillations of the mercury column were visible. This allows the pulse to be studied in terms of its strength, regularity and rhythm. Herisson described the relationship between the accentuated pulse, left ventricular hypertrophy, and apoplexy, but he did not measure blood pressure.
Herisson's sphygmometer [ 9 ].
In 1855, Karl von Vierordt [ 10 ] formulated the principle that “the blood pressure in a vessel can be determined by the external counterpressure necessary to stop the pulsation in the artery”. Based on this still valid principle, he constructed a complicated mechanical device and was the first to attempt to measure blood pressure in a non-invasive way. He determined the arterial BP value in the radial artery. The counterpressure was estimated using weights placed on the lever bowls of the sphygmomanometer connected for recording to the kymograph ( Fig. 1b ).
Vierordt's sphygmograph [ 10 ].
Another researcher who was looking for the possibility of non-invasive blood pressure measurement was Samuel Siegfried Karl Ritter von Basch. In 1880, he constructed an apparatus [ 11 ] similar to the Herisson's sphygmometer ( Fig. 2a ). The BP was measured by applying the pressure until the pulse disappeared in the radial or temporal artery. For portability and practical clinical use, von Basch replaced the mercury manometer with an aneroid manometer ( Fig. 2b ). In the days of mechanistic sphygmomanometry, the diastolic pressure was not determined; only one pressure value, considered the highest (systolic) pressure, or mean blood pressure, was measured.
Von Basch's sphygmomanometer [ 11 ].
Von Basch's aneroid sphygmomanometer [ 11 ].
The first BP measurements in children and newborns
Karl von Vierordt in his monograph “Physiologie des Kinderalters”, published in 1881 [ 10 ], discussed possible differences in blood pressure values during ontogenesis. Using data from other authors, such as Hofmann in 1877 [ 12 ], who measured BP of different animal species and different ages, he concluded that younger animal individuals have lower arterial blood pressure. Since the value of arterial blood pressure in adult pathologic patients determined invasively by Faivre was around 120 mm Hg, he estimated the value of BP of a healthy adult to be around 200 mm Hg. With this assumption, he calculated that the blood pressure of human newborns could be 111 mm Hg, three-year-old children 138 mm Hg and in 14-year-old individuals 171 mm Hg.
These data were taken up by his son, the physiologist Hermann Vierordt, who published an extensive monograph in Jena in 1893 [ 13 ]. In addition to the taken up data from the previously mentioned monograph, he also presented values of blood pressure in the capillaries of the nail bed, changes in BP in the standing position, etc. Interestingly, H. Vierordt also reported blood pressure values (44 and 56 mm Hg) in 4.5 and 10 year old boys in the a. radialis measured by Zadek [ 14 ], as well as in the temporal artery in 2–5 year old children with a value of 97–104 mm Hg measured by Eckert [ 15 ] using von Basch's sphygmomanometer.
The measurements reported by Zadek and Eckert are probably the first non-invasive blood pressure measurements in children. Zadek, who obtained priority results in blood pressure variability, also confirmed the suitability of using the von Basch's sphygmomanometer using experiments on dogs by simultaneous invasive and noninvasive blood pressure measurements.
Another device used to measure blood pressure at the turn of the 19 th and 20 th centuries was the Gärtner's tonometer [ 16 ]. It was an instrument for measuring the finger BP of an adult by determination of the pressure, expressed by the height of the column of mercury, required to stop the artery pulsation in the finger surrounded by a compressive inflatable ring-cuff ( Fig. 3 ). The decreasing pressure value at which blood flow reappeared in the finger was also taken into account. Gärtner thus gave the basis for the flush method.
Gärtner's tonometer [ 16 ].
In 1902 Neu [ 17 ], and in 1906 Trumpp [ 18 ] found with Gärtner's tonometer that the systolic pressure in newborns is 75–90 mm Hg. Sladkoff [ 19 ], cited by Gundobin [ 20 ], used this type of tonometer to measure the BP of 600 children (both newborns and children up to the age of 15 years). He found the average value of 61 mm Hg for the blood pressure of newborns in the first 24 h. He also described an increase in BP with age, especially in the early days and weeks, with a slower rate of rise later in infancy.
Flush method
The principle of this method is based on visualizing the return of blood flow by monitoring the color of the finger, forearm, or leg and correlating it with the decreasing pressure in the occluding cuff. The procedure for measuring blood pressure in the extremities of newborns and infants was independently modified in 1952 by Cappe and Pallin [ 21 ] and Goldring and Wohltman [ 22 ].
Partial ischemization of the finger, upper or lower limb was achieved by gradually squeezing the blood out of the hand or foot by wrapping an elastic bandage or soft rubber starting from the acral parts of the limb. To facilitate drainage, the limb was held above the level of the heart. In more recent blood pressure measurements using this method, Ribeiro et al. 2011 [ 23 ], used gentle hand compression by the examiner to induce ischemia in the hands of newborns until the skin became pale. However, other authors did not consider this method of ischemia to be sufficient [ 24 ].
An occlusion cuff of a blood pressure monitor was then placed over the ischemic part of the limb, inflated to a pressure above the predicted systolic pressure (90–130 mm Hg in newborns).
After the blood flow was stopped, the bandage or rubber band was removed, the limb was kept at heart level, and the pressure in the cuff was slowly reduced at a rate of 4–6 mm Hg every 3–5 s. Simultaneously, the color of the limb and the height of the mercury column were observed to determine the point at which the color changed. The first indication that the blood flow was about to reappear, known as the “flush point”, was the initiation of small oscillations in the mercury column of the tonometer. The maximum oscillations approximately coincided with the height of the mercury column and with the mean arterial pressure [ 25 ].
To make the measurement more accurate, two observers were required: one operated the inflation and deflation of the cuff and read the pressure values, while the other observed the flush – the onset of perfusion. The results obtained using this method were limited by edema, hypothermia, and severe anemia [ 26 ].
The flush method was mainly used in the middle of the 20 th century. Ribeiro et al . [ 23 ] compared three non-invasive techniques for measuring neonatal BP – the flush method, measurement using pulse oximetry, and the oscillometric method. They concluded that the flush method is relatively accurate and may be useful, especially when blood pressure cannot be accurately detected by the oscillometric method. Despite this statement, the number of available scientific papers using the flush method for measuring blood pressure in newborns has significantly declined after the year 2000.
Palpation method
At the turn of the 19 th and 20 th centuries, the palpation method for measuring blood pressure became prominent. Scipione Riva-Rocci in 1896 [ 27 ] used a pneumatic cuff connected to a mercury column, the value of which he increased until the disappearance of the pulse at the a. radialis ( Fig. 4 ). By palpation of the a. radialis and subjective assessment, one detected the moment, and thus the value of systolic pressure, when the pulse disappeared or reappeared as the pressure increased or decreased over or below systolic pressure. The greatest improvement in Riva-Rocci's method of blood pressure measurement was the application of a cuff around the arm, which evenly applied pressure around the limb. This eliminated the most significant error associated with uneven compression of the radial artery using pellets of different sizes. However, the palpation method could not determine diastolic and mean blood pressure, and Riva-Rocci used only a narrow (5 cm) cuff in adults. This error was corrected by von Recklinghausen [ 28 ] as early as 1901. By repeated measurements in adult humans, he showed that narrower cuffs provide higher BP values and increasing the cuff width to 12–14 cm no longer affects the measured BP values.
Measurement of systolic blood pressure by the RR method [ 27 ].
The use of the palpation method in newborns was mainly limited by the problem of pulse palpation at the fine thin a. radialis. Nevertheless, several authors have also used this method in newborns with a preference for palpation of the a. brachialis in the fossa cubiti just below the occlusion cuff.
Reis and Chaloupka in 1923 [ 29 ] measured blood pressure by the palpation method on the first and tenth day after birth and found relatively low values – on the first day after birth the mean systolic pressure was 43 (32–58) mm Hg, on the tenth day 78 mm Hg. However, they used too wide cuff in the measurements, which caused artificially low values of measured BP. They found that after deliveries using forceps, traumatized newborns had a higher BP; after cesarean delivery, BP did not differ from that of newborns delivered physiologically.
The issue of cuff width factor was again highlighted by Woodbury et al . [ 4 ], who found a close correlation between BP values obtained in newborns by the invasive method (measurement in the a. umbilicalis) and the palpation method, but only when a cuff width of 2.5 cm was used.
In the mid-twentieth century, neonatal BP was measured by the palpation method by Holland and Young [ 30 ]. They used a 2.5 cm wide cuff and palpation of the pulse at the a. brachialis just below the occlusion (in the fossa cubiti). They calculated the mean of 2–3 systolic BP measurements, which were measured within 1 h after birth and in the later period of life until the 6 th month of age. In healthy newborns, the mean BP at birth was 69 mm Hg, and at the 6 th month it was 93 mm Hg. Systolic BP in preterm infants was lower than in term infants.
Methods based on sound detection
Significant improvements in blood pressure measurement and the determination of diastolic pressure were made by N. S. Korotkov (1874–1920). He published his discovery of the auscultatory method in 1905 in one of the shortest classical scientific papers in the world, one page in length [ 31 , 32 ].
The auscultatory method has also been used in newborns [ 33 ]. However, this method without determining the optimal size of the occlusion inflation cuff and for morphological/functional reasons (small arm circumference, vessels, as well as oscillation amplitude) gave only variable values. The obtained BP values were usually lower than those obtained by direct measurement [ 34 , 35 ]. The use of the auscultatory method of measuring blood pressure in newborns and infants may also be hindered by uncontrollable limb movements that may accompany cuff inflation and deflation. Therefore, measurement of BP by auscultatory technique in children from birth to approximately 3 years often fails.
Therefore, physiologists, physicians and engineers, focused on detecting the dynamic state of the vessel under the occluding cuff using the Doppler effect.
In 1957, Shigeo Satomura [ 36 ] used the Doppler effect to diagnose cardiovascular anomalies in humans. McCutcheon and Rushmer [ 37 ] proposed the use of ultrasound to detect arterial pulsations, and in 1968, Stegal et al . [ 38 ] constructed an ultrasonic tonometer for measuring BP in adult humans, which could also be used in infants and newborns.
Blood pressure measured using an ultrasound device (e.g. Arteriosonde) has a good correlation with the “gold standard” – values obtained from intra-arterial measurements, especially for systolic blood pressure [ 39 , 40 ], even in newborns [ 23 ]. There has been a proven close correlation with systolic pressure values, but determining diastolic pressure was not so reliable.
Czechoslovak scientists and technicians also contributed to the development of blood pressure measurement in newborns using ultrasound methods. The LUD-802 Tesla ultrasonic tonometer was constructed and patented in Tesla Valašské Meziříčí [ 41 ]. The set included a portable ultrasound device, a miniature probe, and tonometric cuffs of various widths for children ( Fig. 5 ). The ultrasonic tonometer was used in several clinical and physiological studies [ 42 , 43 ]. Accurate evaluation of blood pressure recordings was made possible by connecting a mercury manometer to an electromanometer in parallel and by recording the output ultrasonic signal [ 44 ] ( Fig. 6 ).
LUD-802 TESLA ultrasound blood pressure measurement set consisting of an ultrasound device with headphones, a miniaturized ultrasound probe that was placed over the artery under the occlusion cuff, a set of cuffs of different sizes and a mercury tonometer [ 45 ].
Original record of blood pressure measurement of a premature newborn using a LUD ultrasound tonometer and electromanometer. Upper curve – respiration, lower curve – sound recording, lower curve – cuff pressure recorded by electromanometer.
Not only ultrasound, but also infrasound, generated by vibration of the vessel wall during occlusion and the subsequent restoration of blood flow, was used for non-invasive measurement of blood pressure in newborns. Detection of infrasonic vibrations of the vessel wall at the time when a bolus of blood enters the occluded collapsed segment after the pressure in the occluding inflation cuff has been reduced below the systolic pressure was detected by the Infrasonde 3000 device. Large vibrations (“flutter”) of the artery wall cease at the time the cuff pressure drops below the diastolic pressure [ 45 ]. A reasonably accurate systolic pressure value was obtained using the Infrasonde, but the diastolic pressure was significantly underestimated [ 46 , 47 ].
Oscillometric methods using mechanical detection of arterial oscillations
Étienne Jules Marey constructed a manometer in 1876 [ 48 , 49 ] consisting of a glass box filled with water, in which a hand was placed and sealed (principle of a plethysmograph). The box was connected to a water reservoir that allowed for increasing or decreasing the pressure in the box. It also included a mercury manometer and a recording device, which used a tambour writing system and paper for kymographic tracing. By increasing the pressure in the plethysmograph, the amplitude of the sphygmogram changed until the pulsations disappeared. Marey noted that maximum oscillations were transmitted to the water when the counter-pressure was at a level that maximally unloaded the vessel walls throughout the heart cycle. In 1969, Poesy et al . (50) confirmed that the point of maximum oscillations actually corresponds to true mean arterial pressure. The device was too complicated, the oscillations were dampened by the physical properties of the mercury and it was unsuitable for measuring blood pressure in newborns and children.
A simple oscillometer for measuring blood pressure, easily applicable to humans, was designed, constructed, and experimentally verified by Hill and Barnard [ 51 ] a year after the publication of the palpation method. In 1897, they published a paper describing the construction of an oscillometer for measuring blood pressure in humans. It consisted of an inflatable cuff, a pump, a three-way tap, and a sensitive metal aneroid gauge with a large pointer ( Fig. 7 ). They observed the movements of the aneroid gauge pointer while increasing the pressure in the cuff above the value of the systolic blood pressure, noting the cessation of gauge oscillations, as well as during pressure reduction when the movements of the pointer reappeared. The largest oscillations indicated the value of the mean blood pressure. The authors verified that the pressure value corresponded to the mean blood pressure by conducting experiments with simultaneous blood pressure registration in the femoral artery and blood pressure measurement using the oscillometer in experimental animals. The authors recommended the use of the sphygmomanometer also in children, suggesting to place the cuff on the thigh to measure the blood pressure in the a. femoralis.
Hill and Barnard sphygmomanometer [ 51 ].
Erlanger [ 52 ] in 1904 improved the Marey's method by attaching a Riva-Rocci cuff around the upper arm and recording the cuff pressure oscillations with a kymograph. Oscillations appeared at the moment when the external pressure dropped below the intravascular pressure, which determined the systolic pressure. Erlanger considered the cuff pressure value at the sudden attenuation of the oscillations to be the diastolic pressure value.
In 1909, Victor Pachon [ 53 ] developed a sophisticated sphygmomanometric oscillometer to measure arterial blood pressure, which was used by physicians until the late 1960s. Pachon used one compartment of the cuff to detect cuff pressure and another compartment for showing cuff pressure ( Fig. 8 ). It was the first device that allowed relatively accurate determination of blood pressure without the use of a stethoscope. At that time, it was believed that the maximum amplitude of oscillation indicated the diastolic blood pressure. Later, it was found that the maximum oscillations corresponded to the cuff pressure, which is equal to the mean arterial pressure.
Original drawing of Pachon oscillometer [ 53 ].
Automatic measurement of BP by a device based on oscillometric technique was first described by Ramsey in 1979 [ 54 ]. A modern modification of the oscillometric method, which is still used in most automatic and semi-automatic electronic pressure gauges, was patented in the USA on 24 January 1984 by Nunn and Beveridge [ 55 ]. It utilizes the assessment of pressure oscillations in the cuff caused by the pulse wave using sensors and software. The oscillations begin well above systolic pressure and continue below diastolic, so that systolic and diastolic pressure can only be estimated indirectly according to some empirically derived algorithm [ 56 ].
The non-invasive methods, including oscillometric methods, began to be used intensively in children and newborns as early as the beginning of the 20 th century, as evidenced by a review of 29 papers published between 1902 and 1926 on the measurement of blood pressure in more than 100 newborns and infants and in several thousand children [ 57 ]. The rapid spread of methods for noninvasive BP measurement is evidenced by the affiliations of the papers. There are papers from France, Austria-Hungary, Italy, Germany, USA, UK, Sweden, as well as China and Russia.
In very small children, the oscillometric method was first used in 1912 by Koessler [ 58 ]; cited by Kafka [ 59 ]. Balard [ 60 – 63 ] as early as 1912 made extensive studies of blood pressure and pulse rate with an oscillometric device in newborns by measuring BP on the upper limb. The first measurement was taken in the first minute after birth and the first cry, then after a quarter of an hour and at the end of the first hour, every two hours for the first 12 h, and then every day until 10 days of age. He found that in newborns the systolic pressure was in the range of 35–55 mm Hg and this increased after the first cry as well as with advancing postnatal age, until the 10 th day after birth was observed. Blood pressure as well as pulse rate were higher in the awake state. Ballard also determined diastolic pressure, which was not elevated in the awake state. He attributed the rise in pressure amplitude in the awake state to the increased work of the heart. He measured the body temperature and found that by lowering the temperature, the heart rate decreased in parallel.
At a time when pressure sensors did not exist, the detection of pressure oscillations in the cuff was a crucial problem. Therefore, in addition to the above methods, other methods have been used to detect changes in vessel oscillations and blood flow by cuff occlusion pressure. Ashworth et al . [ 64 ] introduced the xylol method . In this method, a tonometric occlusive cuff was applied to the arm, and a second low-pressure cuff was applied to the wrist area to detect radial artery oscillations. The tube connected to the lower cuff was filled with colored xylol, which began to pulsate with the appearance of a pulse in the radial artery. At that moment, the value of the systolic pressure was read from the mercury manometer connected to the occluding tonometric cuff. The authors reported that the error of this method was ± 4 mm Hg. This method has not found widespread use in clinical practice; it has been used mainly in clinical-physiological studies. For example, Ashworth and Neligan [ 65 ] found by the xylol method that newborns in the first hours after birth when the umbilical cord was ligated during delivery had a lower systolic BP (by 10 mm Hg), compared with newborns whose cord was tied later after birth.
Impedance plethysmography (reoplethysmo-graphy) for measuring systolic pressure in newborns has been used by Schaffer [ 66 ]. Impedance plethysmography records changes in tissue impedance caused by blood flow. After the disappearance of blood flow distal to the occluding cuff after occlusion, the tissue impedance changes cease, and the rheographic curve reappears after the return of flow. Lang and Hilber [ 67 ] used electrical impedance plethysmography in the limbs of newborns and infants to determine the optimal width of inflatable cuffs.
Celander and Thunell [ 68 ] used a volumetric plethysmographic method to measure blood pressure in newborns. Changes in limb volume over a short period of time depend on arterial inflow (and venous outflow). When volume is sensed distally below the occlusive cuff, the first increment of the first arterial inflow determines the systolic pressure value. With a gradual decrease in occlusion pressure, the arterial inflow gradually increases, and the appearance of a maximal inflow given by the cuff pressure value indicates the diastolic pressure value.
Mason and Braunwald [ 69 ] used a strain gauge method in adult humans to sense changes in limb volume and thus to measure both blood flow and pressure in the limbs. The sensor was made of an elastic tube filled with mercury placed around the limb. By changing the length and cross-section due to changes in the volume of the limb by inflow and outflow of blood, the electrical resistance values in the sensor were also changed. This method was also attempted by Gundersen and Dahlin [ 70 ] to measure the blood pressure of newborns. They constructed a miniaturized cuff and sensor with placement on the finger of the hand. The values correlated with the invasive measurements mainly in term newborns weighing more than 3000 g.
A simpler and more accurate method was found to be pulse oximetry [ 71 , 72 ], which was used to determine systolic blood pressure. During cuff inflation, deflation, or both maneuvers, the oximetric waveform dependent on blood flow to the monitored distal part of the limb disappeared and reappeared. These studies using pulse oximetry technique in newborns showed good correlation with direct invasive monitoring, even significantly better compared to values obtained with oscillometric devices. According to Movius et al . [ 71 ], pulse oximetry provides an alternative non-invasive option for measuring blood pressure in newborns and children without the need for expensive equipment, especially in cases where the oscillometric method is inaccurate due to very low blood pressure.
Automatic oscillometric blood pressure measurement
In 1965, Gupta and Scopes published a paper [ 73 ] on an automatic oscillometric device that allowed the systolic blood pressure of newborns to be measured and recorded in a non-invasive manner every two minutes for up to 12 h. The device was based on the oscillometric principle with two cuffs: the upper cuff was occluded, the lower cuff inflated to 10 mm Hg was for the detection of vessel oscillations. The pulsations of the vessel wall and the resulting pressure and air volume changes in the lower cuff were detected by a heated thermistor changing the resistance value by temperature changes by moving cooler air through a thin capillary. The electrical signal was transmitted to the recorder. The authors used this device to gain insights into the physiological changes in BP during bottle-feeding, breastfeeding, pacifier use, and changes in body position.
Currently, even in newborns and infants, the most widely used monitor is the Dinamap monitor using cuff pressure oscillation assessment by a sensitive sensor and special software. The original Dinamap oscillometric monitor was an automated oscillotonometer using two cuffs. Modern systems use only one cuff performing both occlusion and sensing functions.
The oscillometric method is popular due to its convenience and ease of use. Comparison of BP values obtained by oscillometric and invasive measurements by meta-analysis of 34 papers [ 74 ] showed that mean BP values correlate best. However, it can be inaccurate, for example, in very low birth weight newborns and in babies with low mean arterial pressure [ 23 ].
Continuous non-invasive blood pressure monitoring
Non-invasive continuous beat-to-beat blood pressure registration is made possible by devices based on the volume-clamp method developed and described by physiologist Jan Peňáz [ 75 ] in 1968. Peňáz's method works on the principle of the „unloaded arterial wall“. Arterial pulsation in a finger is detected by a photoplethysmograph under a pressure cuff. The output of the plethysmograph is used to drive a servo-loop, which rapidly changes the cuff pressure to keep the output constant, so that the artery is held in a partially opened state. The cuff pressure is recorded and the obtained values of instantaneous beat-to-beat blood pressure and heart rate are stored in the device's memory, transferred to a computer, and processed by special software [ 76 ]. This method gives an accurate estimate of the changes of systolic and diastolic pressure when compared to brachial artery pressure. The Finapres (Ohmeda) device was constructed on this principle, later Finometer, Portapres, Finometer NOVA (FMS) and others.
Their use is appropriate in research, diagnosis and monitoring of adult and pediatric patients. Jagomägi et al . [ 77 ] and Lemson et al. [ 78 ] compared the Finapres measurements with the oscillometric method and concluded that each of these methods shows relevant arterial pressure values.
Studies by Drouin et al . [ 79 ], Andriessen et al. [ 80 ], Yiallorou et al. [ 81 ] demonstrated that even in newborns this method can be applied in a modified way (by placing the cuff on the newborn's wrist instead of the finger). The relevance and validity of such a modified method has been verified and proven by comparison with the invasive method after catheterization [ 80 ].
In newborns, this methodology is mainly used in clinical physiological studies dealing with short-term beat-to-beat blood pressure and heart rate variability, baroreflex sensitivity and rapid blood pressure reactions to various manoeuvres, stimulations, apnoeic pauses, etc. [ 81 – 87 ].
There are no reports yet on the use of other methods under development for non-invasive continuous blood pressure monitoring, such as applanation tonometry, in newborns and young children.
Conclusions
The development of methods for measuring blood pressure in adults and later in newborns was determined by the inventions of physicians and physiologists in the field of medicine, as well as engineers on whom technological progress depended (mechanics, electricity, semiconductors, sensors, computers). Multidisciplinary collaboration was crucial in achieving the current state of having different methods for measuring systemic blood pressure in newborns.
The measurement and obtained blood pressure values provide important information about the functional state of the cardiovascular system. However, this information does not inform about the critical perfusion of individual organs, which may differ from the predicted values by regional regulations and the actual state of the organ. Therefore, other methodologies are currently being developed to detect organ perfusion and their oxygenation status, such as new Doppler ultrasound techniques, near-infrared spectroscopy (NIRS), and others. However, these techniques are more complicated and costly, so the measurement and monitoring of blood pressure in humans, even in newborns and young children, remains of clinical and scientific importance.
Conflict of Interest: There is no conflict of interest.
- 1. Hales S. Statical essays: containing Haemastaticks. An account of some hydraulick and hydrostatical experiments made on the blood and blood vessels of animals. Royal Society of London. 1733;2:1–385. doi: 10.5962/bhl.title.106596. [ DOI ] [ Google Scholar ]
- 2. Faivre J. Études experimentales sur les lésions organiques du coeur. Imprimerie d’Aimé Vingtrinier; Lyon: 1856. pp. 1–54. [ Google Scholar ]
- 3. Haselhorst G. Zum plazentaaren Kreislauf unter der Geburt. Zeitschrift fur Geburtshilfe und Gynäkologie. 1929;95:32–42. [ Google Scholar ]
- 4. Woodbury RA, Robinow M, Hamilton F. Blood pressure studies on infants. Am J Physiol. 1938;122:472–479. doi: 10.1152/ajplegacy.1938.122.2.472. [ DOI ] [ Google Scholar ]
- 5. Robinow M, Hamilton WF, Woodbury RA, Volpitto PP. Accuracy of clinical determinations of blood pressure in children with values under normal and abnormal conditions. Am J Dis Child. 1939;58:102–118. doi: 10.1001/archpedi.1939.01990070114011. [ DOI ] [ Google Scholar ]
- 6. Wallgren G, Karlberg P, Lind J. Studies of the circulatory adaptation immediately after birth. Acta Paediatr. 1960;49:843–849. doi: 10.1111/j.1651-2227.1960.tb16094.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 7. Rudolph AM, Drorbough JE, Auld PM, Rudolph AJ, Nadas AS, Smith CA, Hubbell JP. Studies on the circulation in the neonatal period. The circulation in the respiratory distress syndrome. Pediatrics. 1961;27:551–566. doi: 10.1542/peds.27.4.551. [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. Todres ID, Rogers MC, Shannon DC, Moylan FM, Ryan JF. Percutaneous catheterization of the radial artery in the critically ill neonate. J Pediat. 1975;87:273–275. doi: 10.1016/S0022-3476(75)80601-9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. Herisson JBES. Measurement of the pulse. The sphygmomanometer: An instrument which makes every action of the arteries apparent to the eye. Lancet. 1834;23:22–27. doi: 10.1016/S0140-6736(02)96308-7. [ DOI ] [ Google Scholar ]
- 10. Vierordt K. Physiologie des Kinderalters. Tübingen. 1881:496. [ Google Scholar ]
- 11. Basch von S. Über die Messung des Blutdruckes am Menschen. Zeitschr Klin Med. 1880;2:79–96. [ Google Scholar ]
- 12. Hofmann E. Über Verblutung aus der Nabelschnur. Oesterr Jahrb Pädiatr. 1877;II:192. [ Google Scholar ]
- 13. Vierordt H. Anatomische Physiologische und Physikalische Daten und Tabellen zum Gebrauche fur Mediciner. Verlag Von Gustav Fischer; Jena: 1893. p. 400. [ Google Scholar ]
- 14. Zadek I. Die Messung des Blutdrucks am Menschen mittels des Basch’schen Apparatus. Z Klin Med. 1880/1881;2:509–551.. [ Google Scholar ]
- 15. Eckert A. Beobachtung aus den Elisabeth-Kinderhospital in St. Petersburgh. Wratsch; 1882. p. 220. [ Google Scholar ]
- 16. Gärtner G. Über einen neuen Blutdruckmesser (Tonometer) Wien Med Woch. 1899;49:1412–1418. [ Google Scholar ]
- 17. Neu M. Experimentalle und Klinische Blutdruckuntersuchungen mit Gärtners Tonometer. Inaug Diss Universitätsbuchhandlung; Heidelberg: 1902. p. 172. [ Google Scholar ]
- 18. Trumpp J. Blutdruckmessungen an gesunden und kranken Säuglingen. Jahrb Kinderheilk. 1906;63:43–59. [ Google Scholar ]
- 19. Sladkoff. Blood Pressure in Children. St Petersburg: 1903. [ Google Scholar ]
- 20. Gundobin . Deutsche Ausgabe. von Rubinstein, Berlin: Allgemeine Med Verlagsanstalt; 1912. Die Besonderheiten des Kindesalters. [ Google Scholar ]
- 21. Cappe BE, Pallin IM. Systolic blood pressure determination in the newborn and infant. Anesthesiology. 1952;13:648–650. doi: 10.1097/00000542-195211000-00014. [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. Goldring D, Wohltmann H. Flush method for blood pressure determination in newborn infants. J Pediat. 1952;40:285–289. doi: 10.1016/S0022-3476(52)80257-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. Ribeiro MAS, Fiori HH, Luz JH, Piva JP, Ribeiro NM, Fiori RM. Comparison of noninvasive techniques to measure blood pressure in newborns. J Pediatria (Rio J) 2011;87:57–62. doi: 10.1590/S0021-75572011000100010. [ DOI ] [ PubMed ] [ Google Scholar ]
- 24. O’Brien ET, O’Malley K. ABC of blood pressure measurement. Infancy and childhood. Br Med J. 1979:1048–1049. doi: 10.1136/bmj.2.6197.1048. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 25. Forfar JO, Kibel MA. Blood pressure in the newborn estimated by the flush method. Arch Dis Child. 1956;31:126–130. doi: 10.1136/adc.31.156.126. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 26. Virnig NL, Reynolds JW. Reliability of flush blood pressure measurements in the sick newborn infant. J Pediatr. 1974;84:594–598. doi: 10.1016/S0022-3476(74)80688-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 27. Riva-Rocci S. Un nuovo sfigmomanometro. Gazzetta Medica di Torino. 1896;50:981–1017. [ Google Scholar ]
- 28. Von Recklinghausen H. Über Blutdruckmessung beim Menschen. Arch Exp Pathol Pharmakol. 1901;46:78–132. doi: 10.1007/BF01977772. [ DOI ] [ Google Scholar ]
- 29. Reis RA, Chaloupka AJ. Blood pressure in the newborn following normal and pathological labor. Surg Gyn Obst. 1923;37:206–211. [ Google Scholar ]
- 30. Holland WW, Young IM. Neonatal blood pressure in relation to maturity, mode of delivery, and condition at birth. Br Med J. 1956;2:1331–1333. doi: 10.1136/bmj.2.5005.1331. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 31. Korotkov NS. To the question of methods of determining the blood pressure. Reports of the Imperial Military Academy (Izvestie Imp Voiennomedicinskoi Akademii) 1905;11:365. [ Google Scholar ]
- 32. Booth J. A short history of blood pressure measurement. Proc R Soc Med. 1977;70:793–799. doi: 10.1177/003591577707001112. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 33. Browne FJ, Dodds GH. The cause of hypertension in pre-eclamptic toxaemia. Lancet. 1936;1:1059–1060. doi: 10.1016/S0140-6736(01)37020-4. [ DOI ] [ Google Scholar ]
- 34. Long M, Dunlop JR, Holland WW. Blood pressure recording in children. Arch Dis Childhood. 1971;46:636–640. doi: 10.1136/adc.46.249.636. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 35. Elseed AM, Shinebourne EA, Joseph MC. Assessment of techniques for measurement of blood pressure in infants and children. Arch Dis Childhood. 1973;48:932–936. doi: 10.1136/adc.48.12.932. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 36. Satomura S. Ultrasonic Doppler method for the inspection of cardiac function. J Acoust Soc Am. 1957;29:1181–1185. doi: 10.1121/1.1908737. [ DOI ] [ Google Scholar ]
- 37. McCutcheon EP, Rushmer RF. Korotkoff sounds in experimental critique. Circulat Res. 1967;20:149–161. doi: 10.1161/01.RES.20.2.149. [ DOI ] [ PubMed ] [ Google Scholar ]
- 38. Stegal MF, Kardon MB, Kemmerer WT. Indirect measurement of arterial blood pressure by Doppler ultrasonic sphygmomanometry. J Apl Physiol. 1968;23:793–798. doi: 10.1152/jappl.1968.25.6.793. [ DOI ] [ PubMed ] [ Google Scholar ]
- 39. Hernandez A, Goldring D, Hartmann AF., Jr Measurement of blood pressure in infants and children by the Doppler ultrasonic technique. Pediatrics. 1971;48:788–794. doi: 10.1542/peds.48.5.788. [ DOI ] [ PubMed ] [ Google Scholar ]
- 40. Janis KM, Kemmerer WT, Hagood CO., Jr Doppler blood pressure measuement in infants and small children. J Pediatr Surg. 1971;6:70–72. doi: 10.1016/0022-3468(71)90672-5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 41. Kellerová E, Kittová M, Kováčik P. Neinvazívna metóda merania krvného tlaku u novorodencov na princípe Dopplerovho fenoménu ultrazvuku. (Article in Slovak) Bratisl Lek Listy. 1978;70:409–418. [ PubMed ] [ Google Scholar ]
- 42. Kittnar M, Kellerová E. The age dependence of some cardiovascular parameters in children. Physiol Bohemoslov. 1978;27:253. [ Google Scholar ]
- 43. Javorka K, Zavarská L’. Zmeny systémového tlaku krvi a kardiorespiračních parametrov u nedonosených novorodencov počas fototerapie. (Article in Slovak) Cesk Pediat. 1990;45:230–232. [ PubMed ] [ Google Scholar ]
- 44. Javorka K, Zavarská L’. Tlak krvi u hypotrofických a nedonosených novorodencov. (Article in Slovak) Cesk Pediat. 1983;38:430–434. [ PubMed ] [ Google Scholar ]
- 45. Kellerova E. Metódy neinvazívneho merania krvného tlaku (in Slovak) In: Javorka K, Buchanec J, Kellerova E, editors. Krvný Obeh Plodov, Novorodencov, Detí a Adolescentov. Osveta, Martin: 1992. pp. 77–86. [ Google Scholar ]
- 46. Edwards RC, Goldberg AD, Bannister R, Raftery EB. The infrasound blood-pressure recorder. A clinical evaluation. Lancet. 1976;2:398–400. doi: 10.1016/S0140-6736(76)92410-7. [ DOI ] [ PubMed ] [ Google Scholar ]
- 47. Savage JM, Dillon MJ, Taylor JFN. Clinical evaluation and comparison of the Infrasonde, Arteriosonde, and mercury sphygmomanometer in measurement of blood pressure in children. Arch Dis Childhood. 1979;54:184–189. doi: 10.1136/adc.54.3.184. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 48. Marey EJ. Travaux du laboratoire de M. Physiologie expérimentale. Vol. 2. Marey; Paris: 1876. Pression et vitesse du sang; pp. 236–371. [ DOI ] [ Google Scholar ]
- 49. Major RH. The history of taking the blood pressure. Ann Med His. 1930:47–55. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 50. Posey JA, Geddes LA, Moore WH. The meaning of the point of maximum oscillations in cuff pressure in the indirect measurement of blood pressure. Cardiovasc Res Cent Bull. 1969;8:15–25. [ PubMed ] [ Google Scholar ]
- 51. Hill L, Barnard H. A simple and accurate form of sphygmometer or arterial pressure gauge contrived for clinical use. Brit Med J. 1897 Oct 2;:904. doi: 10.1136/bmj.2.1918.904. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 52. Erlanger J. A new instrument for determining the minimum and maximum blood-pressures in man. Johns Hopkins Hosp Rep. 1904;12:53–100. [ Google Scholar ]
- 53. Pachon V. Exposé des titres et travaux scientifiques. Paris: Libraires de l’Academie de Medicine; 1911. p. 122. [ Google Scholar ]
- 54. Ramsey M. Noninvasive automatic determination of mean arterial pressure. Med Biol Eng Comput. 1979;17:11–18. doi: 10.1007/BF02440948. [ DOI ] [ PubMed ] [ Google Scholar ]
- 55. Nunn DE, Beveridge RW. Apparatus and method for measuring blood pressure. 4427013 Patent N. 1984 January 24;
- 56. Jílek J, Štork M. Oscilometrické monitory krevního tlaku: metody měření, validace a koncept databáze oscilometrických tlakových vln. (in Czech) Lékař a Technika. 2004;35:160–164. doi: 10.3200/REVU.35.4.160-164. [ DOI ] [ Google Scholar ]
- 57. References on the physical growth and development of the normal child. Bureau Publ. N. 179. Edit. US Dept. Of Labor and US Children’s Bureau. US Government Printing Office; Washington: 1927. p. 355. [ Google Scholar ]
- 58. Koessler L. Thése. Paris: 1912. L’oscillométrie appliqué a l’étude de la tension artérielle chez les enfants; p. 192. [ Google Scholar ]
- 59. Kafka HL. Blood pressure measurement in infants. Arch Dis Child. 1974;49:970. doi: 10.1136/adc.49.12.970. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 60. Balard P. Modifications évolutives du pouls et de la tension artérielle chez le nouveau-né, dans le premiers jours de la vie, étudiées par l’oscillométrie. Compt Rend Hebd Séances Memoires Soc Biol Paris. 1912a;73:483–485.. [ Google Scholar ]
- 61. Balard P. Sur la cause de la diminuation de frequence du pouls chez le nouveau-né dans le premiérs heures de la vie. Vol. 73. Compt Rend Hebd Séances Memoires Soc Biol; Paris: 1912b. pp. 486–488. [ Google Scholar ]
- 62. Balard P. Le pouls et la tension artérielle de l’enfant et du nouveau-né. Gazette des hopitaux, Paris. 1913;86:837–841. [ Google Scholar ]
- 63. Balard P. La tension artérielle et l’oscillométrie chez le nouveau-né. Nourisson Paris. 1921;9:304–319. [ Google Scholar ]
- 64. Ashworth AM, Neligan GA, Rogers JE. Sphygmomanometer for the newborn. Lancet. 1959;1:801–803. doi: 10.1016/S0140-6736(59)91994-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- 65. Ashworth AM, Neligan GA. Changes in the systolic blood pressure of normal babies during the first twenty-four hours of life. Lancet. 1959;1:804–807. doi: 10.1016/S0140-6736(59)91995-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 66. Schaffer AI. Neonatal blood pressure studies. AMA Am J Dis Child. 1955;89:204–209. doi: 10.1001/archpedi.1955.02050110246012. [ DOI ] [ PubMed ] [ Google Scholar ]
- 67. Lang VO, Hilber HM., Jr Zur Blutdruckmessung im Säuglingsalter. Z Kinder-Heilk. 1969;105:156–164. doi: 10.1007/BF00438937. [ DOI ] [ PubMed ] [ Google Scholar ]
- 68. Celander O, Thunell G. A plethysmographic method for measuring the systolic and diastolic blood pressure in newborn infants. Acta Paediatr. 1960;49:497–502. doi: 10.1111/j.1651-2227.1960.tb07764.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 69. Mason DT, Braunwald E. A simplified plethysmographic system for the measurement of systemic arterial pressure and peripheral blood flow. Amer Heart J. 1962;64:796–804. doi: 10.1016/0002-8703(62)90178-3. [ DOI ] [ Google Scholar ]
- 70. Gundersen J, Dahlin K. Measurement of systolic blood pressure in fingers of newborn infants. Acta Paediatr Scand. 1975;64:741–744. doi: 10.1111/j.1651-2227.1975.tb03913.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 71. Movius AJ, Bratton SL, Sorensen GK. Use of pulse oximetry for blood pressure measurement after cardiac surgery. Arch Dis Child. 1998;78:457–460. doi: 10.1136/adc.78.5.457. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 72. Langbaum M, Eyal FG. A practical and reliable method of measuring blood pressure in the neonate by pulse oximetry. J Pediatr. 1994;125:591–595. doi: 10.1016/S0022-3476(94)70016-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 73. Gupta JM, Scopes JW. Observations on blood pressure in newborn infants. Arch Dis Childhood. 1965;40:637–644. doi: 10.1136/adc.40.214.637. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 74. Dionne JM, Bremner SA, Baygani SK, Batton B, Ergenekon E, Bhatt-Mehta V, Dempsey E, et al. Method of Blood Pressure Measurement in Neonates and Infants: A Systematic Review and Analysis. J Pediatr. 2020;221:23–31. doi: 10.1016/j.jpeds.2020.02.072. [ DOI ] [ PubMed ] [ Google Scholar ]
- 75. Penaz J. Photoelectric measurement of blood pressure, volume and flow in the finger. Dresden. Digest 10th Int Conf Med Biol Engng; 1973; p. 104. [ Google Scholar ]
- 76. Wesseling KH, Wit de B, Hoeven GMA, Goudoever J, Settels JJ. Physiocal, calibrating finger vascular physiology for finapres. Homeostasis Health Dis. 1995;36:67–82. [ Google Scholar ]
- 77. Jagomäki K, Talts J, Raamat R, Länsimies E. Continuous non-invasive measurement of mean blood pressure in fingers by volume - clamp and differential oscillometric method. Clin Physiol. 1996;16:551–560. doi: 10.1111/j.1475-097X.1996.tb01020.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 78. Lemson J, Hofhuizen CM, Schraa O, Settels JJ, Scheffer G-J, Van der Hoeven JG. The reliability of continuous noninvasive finger blood pressure measurement in critically ill children. Anesth Analg. 2009;108:814–821. doi: 10.1213/ane.0b013e318194f401. [ DOI ] [ PubMed ] [ Google Scholar ]
- 79. Drouin E, Gournay V, Calamel J, Mouzard A, Rozé JC. Feasibility of using finger arterial pressure in neonates. Arch Dis Child Fetal Neonatal Ed. 1997;77:F139–F140. doi: 10.1136/fn.77.2.F139. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 80. Andriessen P, Schoffelen RL, Berendsen RC, de Beer NA, Oei SG, Wijn PF, Blanco CE. Noninvasive Assessment of Blood Pressure Variability in Preterm Infants. Pediatr Res. 2004;55:220–223. doi: 10.1203/01.PDR.0000104152.85296.4F. [ DOI ] [ PubMed ] [ Google Scholar ]
- 81. Yiallourou SR, Walker AM, Horne RS. Validation of a new noninvasive method to measure blood pressure and assess baroreflex sensitivity in preterm infants during sleep. Sleep. 2006;29:1083–1088. doi: 10.1093/sleep/29.8.1083. [ DOI ] [ PubMed ] [ Google Scholar ]
- 82. Andriessen P, Oetomo SB, Peters C, Vermeulen B, Wijn PF, Blanco CE. Baroreceptor reflex sensitivity in human neonates: The effect of postmenstrual age. J Physiol. 2005;568:333–341. doi: 10.1113/jphysiol.2005.093641. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 83. Yiallourou SR, Sands SA, Walker AM, Horne RS. Postnatal development of baroreflex sensitivity in infancy. J Physiol. 2010;588:2193–2203. doi: 10.1113/jphysiol.2010.187070. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 84. Fyfe KL, Yiallourou SR, Wong FY, Odoi A, Walker AM, Horne RS. Gestational age at birth affects maturation of baroreflex control. J Pediat. 2015;166:559–565. doi: 10.1016/j.jpeds.2014.11.026. [ DOI ] [ PubMed ] [ Google Scholar ]
- 85. Haskova K, Javorka M, Czippelova B, Zibolen M, Javorka K. Baroreflex sensitivity in premature infants - relation to the parameters characterizing intrauterine and postnatal condition. Physiol Res. 2017;66(Suppl 2):S257–S264. doi: 10.33549/physiolres.933681. [ DOI ] [ PubMed ] [ Google Scholar ]
- 86. Javorka K, Haskova K, Czippelova B, Zibolen M, Javorka M. Baroreflex sensitivity and blood pressure in premature infants - dependence on gestational age, postnatal age and sex. Physiol Res. 2021;70(Suppl 3):S349–S356. doi: 10.33549/physiolres.934829. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 87. Javorka K, Haskova K, Czippelova B, Zibolen M, Javorka M: Blood pressure variability and baroreflex sensitivity in premature newborns-an effect of postconceptional and gestational age. Front Pediatr. 2021;9:653573. doi: 10.3389/fped.2021.653573. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (1.4 MB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
10 Times Well-Loved Scientists Were Total Jerks
6. university of california - experimenting on newborns.

As if frightening the life out of orphans wasn't bad enough, researchers at the University of California began an experiment in the 1960s, looking into changes in blood pressure and blood flow ... on newborn babies.
The 113 test subjects ranged from one hour to three days old and were the subject of a series of experiments that were, if not dangerous, pretty mean.
The experiments ranged from dunking the babies' feet in ice water to pushing a needle through the umbilical arteries all the way to the heart.
Then, just because they thought they hadn't quite achieve optimum jerk levels yet, in a separate experiment, someone had the bright idea of strapping 50 newborns to a tilting table so that all the blood would rush to their heads.
None of the infants appear to have been harmed by the experiments, but it's a pretty rough way to spend your first couple of days on earth.
Writer. Raconteur. Gardeners' World Enthusiast.
50 Years Ago in The Journal of Pediatrics: General Pediatrics: A Study of Practice in the Mid-1960's
Affiliation.
- 1 Department of Pediatrics University of California, San Francisco San Francisco, California.
- PMID: 30049401
- DOI: 10.1016/j.jpeds.2018.01.057
Publication types
- Historical Article
- History, 20th Century
- Pediatrics / history*
- Periodicals as Topic / history*
- Primary Health Care / history*

COMMENTS
The blood pressure of newborn infants in asphyxial states and in hyaline membrane disease. Pediatrics. 1960 Nov; 26:735-744. [Google Scholar] RUDOLPH AM, DRORBAUGH JE, AULD PA, RUDOLPH AJ, NADAS AS, SMITH CA, HUBBELL JP. Studies on the circulation in the neonatal period. The circulation in the respiratory distress syndrome.
Systolic and diastolic blood pressures have been determined in 20 infants by the use of an automatic blood pressure-recording machine. The mean systolic blood pressure was 7.1 cm Hg (range 5.8 to 9.5). The mean diastolic blood pressure was 5.2 cm Hg (range 4.2 to 6.4).
This is a PDF-only article. The first page of the PDF of this article appears above.
Children were the raw material of medical research - CBS 60 Minutes /Newborn Screening for 29 conditions - NYT . Mon, 28 Feb 2005 . Children have historically been the voiceless victims of medical research abuse - and the doctors and staff who abused them have almost never been held accountable - they are shielded by a whitewashed wall of silence.
Schaffer AJ 1960 Diseases of the Newborn. WB Saunders, Philadelphia. ... Tooley WH 1969 Aortic blood pressure in normal newborn infants during the first 12 hours of life.
Experiments on Newborns In the 1960s, researchers at the University of California began an experiment to study changes in blood pressure and blood flow. The researchers used 113 newborns ranging in age from one hour to three days old as test subjects. In one experiment, a catheter was inserted through the umbilical arteries and into the aorta.
An occlusion cuff of a blood pressure monitor was then placed over the ischemic part of the limb, inflated to a pressure above the predicted systolic pressure (90-130 mm Hg in newborns). After the blood flow was stopped, the bandage or rubber band was removed, the limb was kept at heart level, and the pressure in the cuff was slowly reduced ...
As if frightening the life out of orphans wasn't bad enough, researchers at the University of California began an experiment in the 1960s, looking into changes in blood pressure and blood flow ...
Blood flow in the foot and calf of the newborn. A plethysmographic study. Blood flow in the foot and calf of the newborn. A plethysmographic study. Blood flow in the foot and calf of the newborn. A plethysmographic study Acta Paediatr (Stockh). 1960 Jul;49:488-96. doi: 10.1111/j.1651-2227.1960.tb07763.x. Author O CELANDER. PMID: 13808744 ...
50 Years Ago in The Journal of Pediatrics: General Pediatrics: A Study of Practice in the Mid-1960's J Pediatr. 2018 Aug:199:64. doi: 10.1016/j.jpeds.2018.01.057. Authors Budd N Shenkin 1 , Michael D Cabana 1 Affiliation 1 Department of Pediatrics University of California ...