

Burette: Types, Uses, Advantages, Limitations
A burette is a crucial laboratory instrument widely employed in quantitative analysis, particularly in industrial chemical tests involving titration. It is instrumental in determining the concentration of unknown solutions by utilizing solutions of known concentration. Burettes play a pivotal role in laboratories by facilitating precise liquid measurement and dispensing. Their primary application lies in titrations, ensuring accurate determination of unknown solution concentrations.
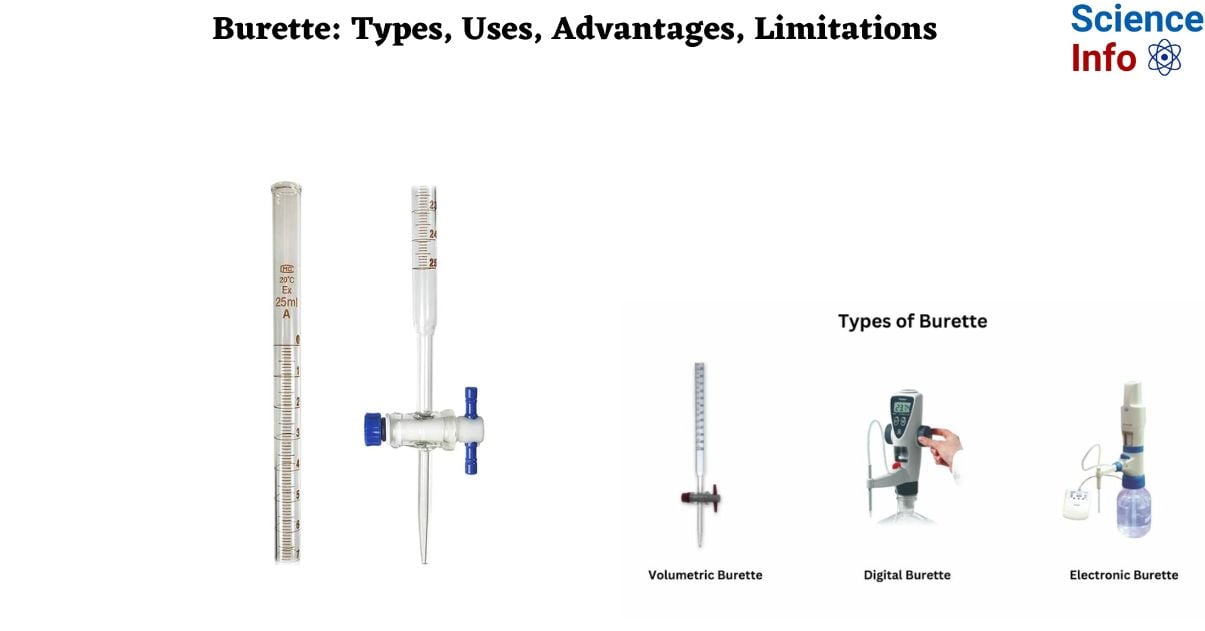
Burettes are versatile tools that find utility in various experimental procedures, including acid-base and redox reactions. Their significance extends to quantitative chemical analysis, making them indispensable in scientific research and educational settings. Additionally, base burette laboratory apparatus uses underscore their integral role in diverse laboratory experiments.
Table of Contents
Interesting Science Videos
What is Burette?
A burette is a laboratory instrument designed for the accurate dispensing of small volumes of liquid or gases. It consists of a long, graduated glass tube equipped with a valve, typically located at one end, allowing precise control of the liquid flow. Functionally akin to a pipette, the burette is an essential tool in quantitative chemical analysis, primarily employed to measure the volume of a liquid or gas in experimental procedures.
Key features of a burette include its graduated markings along the length of the glass tube, enabling precise volume readings. The stopcock, positioned at the lower end, regulates the release of the liquid, facilitating controlled dispensing either in a continuous stream or dropwise. This instrument finds widespread use in titration processes, a methodical approach to determining the quantity of a specific constituent in a given sample by introducing a precisely measured quantity of another substance.

The term “burette” was coined by the renowned French chemist Joseph Louis Gay-Lussac in 1824. Its invention is credited to French chemist Etienne Ossian Henry in 1845, with subsequent improvements introduced by German scientist Karl Friedrich Mohr in 1855. The historical evolution of the burette underscores its crucial role in scientific experimentation. The liquid burette allows for the precise measurement of dispensed volumes, while the gas burette, with the stopcock at the top, measures gas volume by displacing a fluid, such as water, oil, or mercury, from the graduated tube.
Types of Burette
Burettes, essential instruments in laboratory settings for precise liquid measurements, come in three distinct types:
- Piston/Digital, and
A comprehensive understanding of each type is paramount for ensuring accuracy in various experiments.
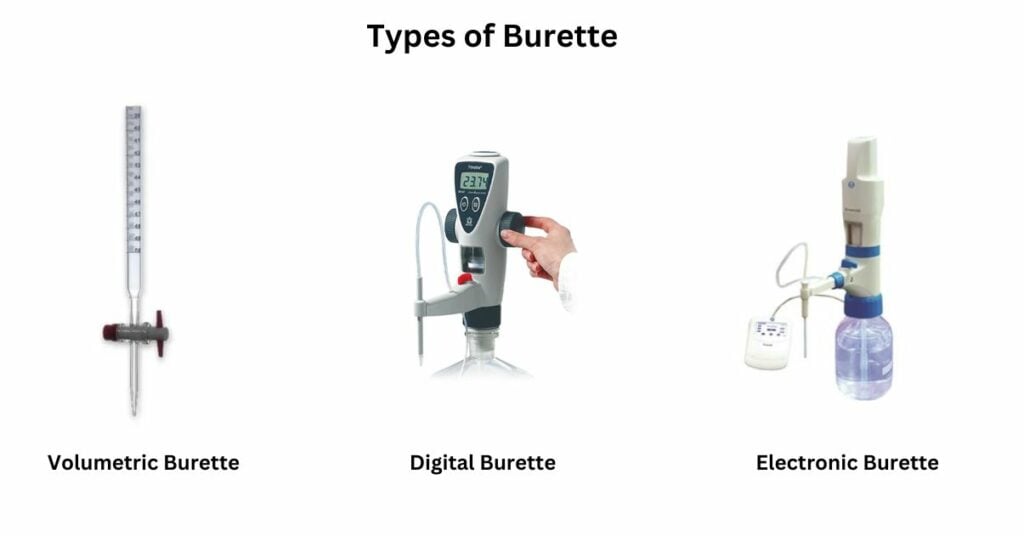
Volumetric Burette
A volumetric burette, typically crafted from glass or plastic, features a printed volume scale on its wall. Equipped with a stopcock at the instrument’s end and a valve for liquid flow control, it serves as a crucial tool in laboratories.
This type further branches into liquid and gas burettes.
Liquid Burette: In the liquid version, a long graduated glass tube with a bottom stopcock allows controlled liquid flow through the force of gravity. The exact liquid volume is determined by reading the markings on the glass tube.
Gas Burette : A gas burette has its stopcock at the upper side, filled with a fluid such as water or mercury. Gas collection occurs by displacing the fluid, and the gas volume is measured by the displaced fluid’s volume.
Piston or Digital Burette
The piston or digital burette adopts a syringe design, with the barrel and plunger typically made of glass or plastic. For alkali solutions, polyethylene or a similarly resistant plastic material may be employed. This type allows for precise liquid delivery through incremental movements of the plunger, either manually by rotating a wheel or via a step motor. A digital display at the top of the burette indicates the volume, providing a high level of accuracy. Motorized versions can be computer-controlled for enhanced precision.
Electronic Burette
The Electronic Burette, or E-Burette, represents the pinnacle of technological advancement in liquid dispensing. It incorporates an electronic display, control panel, and motor-driven piston movements, eliminating manual dispensing challenges present in digital burettes. The precision of the electronic burette surpasses that of its digital counterpart due to motor-controlled dispensing. This automated system eradicates human errors from the titration/dispensing process, elevating the accuracy of titrations to new heights.
Parts/Components of Burette
Volumetric Burette : A Volumetric or glass burette consists of a graduated glass tube with a stopcock at one end, allowing for precise liquid dispensing. Volume markings on the glass tube enable accurate measurement, and a dispensing tip at the opposite end aids in controlled liquid release. The stopcock, rotatable in both directions, governs the flow of liquid.
Electronic Burette : The Electronic Burette, or E-Burette, is an advanced electronic device designed for accurate titration while minimizing the risk of chemical exposure. It incorporates several sophisticated components, including:
- Recirculation Valve: Prevents reagent loss during purging by redirecting it back into the bottle.
- Delivery Tube: Facilitates reagent delivery beyond the reservoir.
- Inspection Window: A transparent or amber-colored acrylic window for light-sensitive chemicals.
- Delivery Nozzle: Provides flexibility for dispensing in diverse laboratory conditions.
- TFT Screen: User-friendly touchscreen for displaying precise data up to the second decimal.
- Telescopic Tube: Adjustable in length, used to draw liquid from the reagent bottle.
- Adapter: Standard sizes (28mm, 32mm, 38mm, 40mm, and 45mm) for fitting various reagent bottles.
- Stylus: A handheld instrument for operating the control panel.
- Control Panel: Employs powerful functions such as automatic refilling, zero reset, and instrument shutdown.
- Middle Housing: Houses the piston mechanism within the burette.

Digital Burette: Distinct from the E-Burette, the Digital Burette shares similar functionalities but features manual piston movement. Components include:
- Recirculation Valve, Delivery Tube, Inspection Window, Telescopic Tube, and Adapters: Similar functionalities as in the Electronic Burette.
- Electrical Display: Eliminates meniscus reading errors, displaying the volume of dispensed liquid.
- Hand Wheel: Allows users to manually set the dispensed liquid amount by rotation.
- Control Buttons: ON/OFF, CLEAR/Selection, and Pause buttons on the upper housing for process control.
How To Use Burette?
A burette is an indispensable tool in laboratories, serving as a cornerstone for precise liquid measurements. Accurate volume measurements are critical in titration operations, thus learning how to use it well is essential for effective experimentation. Burettes are available in various sizes, ranging from 5ml to 100ml. The common feature across these sizes is the presence of a stopcock at the end of the glass apparatus.
- Securing the Burette: Begin by securing the burette in a burette clamp attached to a ring stand. Proper setup is fundamental to the accurate operation of the instrument.
- Preliminary Rinsing: Rinse the burette two or three times with the liquid of choice. Pour a small amount, rotate horizontally, and allow the liquid to pass through the stopcock into a waste container.
- Filling the Burette: Fill the burette with the intended liquid for dispensing. Read the volume, noting that precise leveling is not mandatory. A piece of paper behind the burette aids in reading, considering the numbering discrepancy with graduated cylinders.
- Controlled Dispensing: Gradually allow the liquid to drain into the receiving vessel, exercising control over the dispensing process.
- Closing the Stopcock: Close the stopcock when the desired liquid amount has been dispensed. Tap the burette against the vessel to remove any lingering drops.
- Volume Recordkeeping: Record the remaining liquid volume in the burette. Calculate the delivered liquid volume by subtracting the initial volume from the final volume, providing a precise measurement.
- Draining Excess Liquid: Drain any excess liquid from the burette.
- Rinsing for Cleaning: Rinse the burette with water to ensure cleanliness and prepare it for subsequent use.

Applications of Burettes in Various Industries
Burette plays a pivotal role in the titration process, offering quantitative analysis in diverse industrial chemical testing scenarios where the concentration of an unknown solution needs determination. The widely employed process of titration finds applications in several industrial sectors, including:
Pharmaceutical Industry
- Purity Analysis: Ensuring the purity of pharmaceutical substances.
- Content Analysis: Verifying the content concentration of medicinal compounds.
- Precipitation Titrations: Utilized in determining precipitate formation in pharmaceutical solutions.
- pH-Stat Titrations: Employed for maintaining constant pH levels in pharmaceutical formulations.
Wine Industry
- Acidity Testing: Determining the acidity levels in wine for quality control.
Automotive Industry
- Quality Control: Ensuring the accuracy of chemical compositions in automotive fluids.
Food and Beverage Industry
- Acidity Testing: Assessing and maintaining optimal acidity levels in various liquid food products.
Cosmetic Industry
- Ingredient Concentration Verification: Confirming the concentration of ingredients within safe limits in cosmetic formulations.
Calibration Procedure for Burette
To execute the calibration procedure for a volumetric buret, ensure you have the necessary equipment: a 50 mL buret, two clean 125 mL Erlenmeyer flasks, and a #5 rubber stopper.
Initial Setup:
- Select a 50 mL buret from the designated cabinet.
- Attach a piece of tape with your name to identify the buret.
- Disassemble the stopcock, clean the bore with warm Alconox solution, and reassemble.
Initial Filling and Reading:
- Fill the buret with distilled water, ensuring no air bubbles are trapped.
- Drain water slowly until the meniscus is at 0.00 mL.
- Record the initial meniscus reading after allowing for drainage and stand for 5 minutes.
- Recheck the reading; if tight, no change should be noticeable. Adjust if needed.
Weighing and Volume Measurement
- Weigh a dry Erlenmeyer flask with a #5 rubber stopper.
- Record the meniscus level, run about 10 mL into the flask at 10 mL/minute, and record the final meniscus level.
- Stopper the flask, weigh it, and record the temperature.
Volume Correction
- Use the table provided to convert the mass of water into the true volume at 20 °C.
- Calculate the correction value for each volume, ensuring it adheres to the tolerance allowed for Class A burets.
- Repeat the procedure for additional 10 mL increments up to 50 mL, noting any deviations.
Graphical Analysis
- Plot correction values vs. apparent volume on a graph for each run.
- Ensure similarity between the two plots for confidence in data reliability.
- Use the average correction value for each pair of readings as the final burette correction.
Proper Maintenance and Storage of Burette
To ensure the optimal performance and longevity of an Automatic Burette, a meticulous cleaning and maintenance routine is essential. Follow these steps for effective care:
Cleaning Process
- Thorough Cleaning: Begin by conducting a comprehensive cleaning of the Automatic Burette.
- Distilled Water Rinse: Rinse the burette meticulously with distilled water twice, ensuring thorough coverage.
- Drain Using Stopcock: Employ the stopcock to drain the rinsed water completely from the burette.
- Solution Rinse: Perform an additional rinse with the specified solution, ensuring the entire internal surface is covered.
- Rolling and Draining: Roll the burette to facilitate even distribution of the solution and then drain it completely.
- Drying : Allow the burette to dry completely before storage.
Storage Guidelines
- Handle the Burette with care, especially during storage.
- Utilize a freestanding rack for secure and organized storage.
- Store the Burette in an inverted position to prevent dust or contaminants from entering.
- Keep the taps open during storage to ensure any residual liquid drains out.
Advantages of Burette
High Precision Volume Measurement: Burettes excel at providing precise measurements of liquid volumes, particularly during titration operations. The graduations on the scale improve precision.
Versatile Applications Across Industry: Burettes are used in a variety of industries, including medicines, food and beverage, and cosmetics. They are versatile and may adapt to different titration requirements.
Controlled and Gradual Liquid Dispensing: The stopcock mechanism in burettes enables regulated and progressive liquid distribution, which is crucial for reaching exact titration endpoints.
Facilitates Quantitative Analysis: Burettes are essential in quantitative analysis through titration, allowing the measurement of unknown solution concentrations using known solutions.
Repeatability and Consistency: Burettes, with correct calibration and handling, can reliably produce repeatable results, increasing the dependability of laboratory tests.
Limitations of Burette
Skill-Based Reading Challenges: Accurate readings from a burette need a certain level of skill and expertise, as parallax errors can reduce measurement precision.
Sensitivity to environmental factors : Burettes are susceptible to environmental variables such as temperature, pressure, and humidity. These factors may have an impact on volume measurement accuracy.
Time-consuming Titration Processes: Titration techniques employing burettes can be time-consuming, especially when handling large sample volumes. This presents issues in laboratories aiming for high throughput.
Potential Risk of Contamination: Contamination hazards develop when adequate cleaning processes are not strictly followed, which can lead to inaccurate data and impaired experimental outcomes.
Fragility and susceptibility to breakage: Burettes, especially ones made of glass, are fragile and prone to breaking. Delicate handling is required to avoid damage and maintain measurement accuracy.
Limited Volume Range: Burettes typically function in specified volume ranges. Instances where essential measurements fall outside of this range may necessitate extra equipment, increasing complexity.
Video on How To Use A Burette
- https://www.microlit.us/what-are-the-use-of-burettes-in-pharmaceutical-industry/
- https://chem.libretexts.org/Ancillary_Materials/Demos_Techniques_and_Experiments/General_Lab_Techniques/Proper_Use_of_a_Buret
- https://www.wiltronics.com.au/wiltronics-knowledge-base/how-burettes-work-explained/
- https://www.rdworldonline.com/what-is-a-burette/
- https://www.glasscolabs.com/product-category/laboratory-glassware/laboratory-glasswares-burettes/
- https://www.globalspec.com/learnmore/labware_scientific_instruments/clinical_research_labware/burettes
- https://www.microlit.us/faqs/how-to-use-a-burette/
- https://www.physicsforums.com/threads/advantages-of-a-burette-vs-measuring-cylinder.11771/
- https://chemed.chem.purdue.edu/genchem/lab/equipment/buret/use.html
- https://www.microlit.us/e-burette-product-guide/
About Author
Jyoti Bashyal
Leave a Comment Cancel reply
Save my name, email, and website in this browser for the next time I comment.
Experimental Procedure:
Before coming to the laboratory
"You are expected to plot a graph of P vs T in order to determine the Vapor Pressure of water at the temperature that you performed the experiment. Even in the unlikely event that that temperature is in fact listed!!
Vapor Pressure of Water as a Function of Temperature

IMAGES
VIDEO
COMMENTS
The historical evolution of the burette underscores its crucial role in scientific experimentation. The liquid burette allows for the precise measurement of dispensed volumes, while the gas burette, with the stopcock at the top, measures gas volume by displacing a fluid, such as water, oil, or mercury, from the graduated tube.
Please consider supporting the channel on Patreon!https://www.patreon.com/SupremeScienceThis video demonstrates how to use a gas buret in the chemistry lab.
the products are typically the salt of the metal plus hydrogen gas. In this experiment, magnesium metal reacts with HCl(aq) according to the following equation: € Mg(s)+2HCl(aq)→MgCl 2 (aq)+H 2 (g) The actual volume of hydrogen gas produced in this reaction can be measured using a gas buret using a technique known as water displacement.
Wear safety glasses throughout the experiment. Flush air from the gas burette as follows: 1. Check that the sample tube has been taken off the line. If it is still in place, remove it by holding the glass above the sample tube and gently pulling and twisting the tube. 2. Cautiously turn on the nitrogen cylinder and adjust the outlet pressure so ...
The Ideal Gas Constant OBJECTIVE: This experiment is designed to provide experience in gas handling methods and experimental insight into the relationships between pressure, volume, temperature and the number of moles of a gas. ... Invert the gas buret into the beaker so that the top of the buret is below the water level in the beaker. Clamp
Determining the Ideal Gas Constant: While this experiment is simple in its design, we do have one problem to address: how to determine the pressure exerted by the H 2 (g). By adjusting the level of water in the Florence flask to match that in the buret, we are ensuring that the pressure inside the buret is equal to atmospheric pressure.
In this experiment, you used a gas buret to measure the volume of hydrogen gas that was evolved when a small piece of magnesium or zinc was reacted with acid. From the volume of hydrogen that was ... (21.1 mm Hg at the temperature of my experiment) and the pressure due to the column of liquid water (2.58 mm Hg as calculated in Part 4 above ...
Burettes are the most accurate way of measuring a variable volume of liquid between 0 cm 3 and 50 cm 3. They are most commonly used in titrations. Careful: Read the burette scale from top to bottom as 0.00 cm 3 is at the top of the column Volumetric pipettes are the most accurate way of measuring a fixed volume of liquid,. They have a scratch mark on the neck which is matched to the bottom of ...
A gas buret or gas collection apparatus is illustrated in Figure 1. 125-mL Erlenmeyer flask (reaction flask) rubber stopper rubber stopper gas buret levelling bulb ... settings, but both involve gas evolution experiments. You will work on one scenario with a partner; therefore, you and your partner should begin planning how to approach and ...
In this experiment, students react magnesium ribbon with dilute hydrochloric acid to produce hydrogen gas. They can then use the measured volume of hydrogen gas produced and the mass of magnesium to calculate the mass of magnesium required to produce one mole of hydrogen molecules. ... Burette, 50 cm 3 (see note 1 below) Burette stand; Funnel ...