
The MagLab is funded by the National Science Foundation and the State of Florida.
- About the MagLab /
- Around the Lab /
- What is that? /

Diamond Anvil Cell

Diamonds are a scientist's best friend — especially if that scientist needs to compress a material.
Pressure is one of the parameters scientists play with at the National MagLab. Researchers come to put various materials into the powerful magnets, and then watch what happens to them when they are subjected to lasers, different temperatures, electrical currents or high pressure.
One way scientists compress materials is to put them in a little contraption called a diamond anvil cell (DAC). "They're nothing more glamorous than a nutcracker," says MagLab physicist Stan Tozer, who specializes in high-pressure experiments.
Make that a nutcracker on steroids.
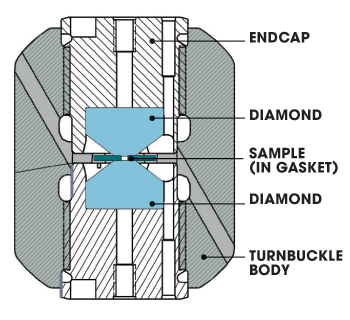
Diagram of a diamond anvil cell.
Many materials behave differently in different environments. Niobium-tin and niobium-titanium, for example, become superconducting at very low temperatures, which is why they are used to build superconducting magnets. These magnets need to sit in a bath of liquid helium to stay cold enough to operate.
The compound cerium-indium 3 (CeIn 3 ) can also become superconducting. For that to happen, though, you don't just freeze it: You squeeze it.
Just as niobium-tin requires ridiculously low temperatures to be superconducting (around -460 degrees Fahrenheit), CeIn3 requires ridiculously high pressures: 2.5 gigapascals (GPa), to be precise. Pascals are a unit of pressure, and 2.5 GPa is about 12,000 times the pressure in a car tire.
How do you safely and effectively bring so much pressure to bear on a tiny sample? Enter Tozer's nutcracker.
Tozer's characterization notwithstanding, diamond anvil cells are kind of glamorous. They feature two diamonds that are purer, clearer and more perfect than any of the rocks in your rings or necklaces. Because they are nature's hardest material, they can effectively smoosh anything you place between them.
Diamonds aren't the only precious gems in the MagLab's jewelry box: Tozer also uses sapphire anvils for experiments at lower pressures and crushed rubies to measure pressure in the cells.
Most of the MagLab's DACs, crafted in our machine shop, use a turnbuckle design devised by Tozer. As shown in the illustration above, two diamonds, encased in the turnbuckle body, face each other pointy side first. In the very tiny space between the points (polished to provide a small, flat surface) sits a gasket into which scientists place an even tinier sample — no wider than a human hair. When the system is closed and the turnbuckle is turned, the endcaps force the two diamonds toward each other, squeezing the devil out of whatever is sandwiched between them.
All of this happens in a package smaller than your pinky.
Scientists have discovered many interesting things by compressing materials. Hydrogen, for example, turns metallic under very high pressure; in fact, Jupiter is believed to be made up in part of condensed, metallic hydrogen. Also, water subjected to varying pressures up to 100 GPa creates 10 different kinds of ice crystals. High pressures could potentially make cars more efficient, as well: Methane forms dense structures that, when squeezed, could allow us to store more energy in our vehicles.
At the MagLab, diamonds are not only gorgeous, but a scientific workhorse, as well. Now that's precious!
Thanks to MagLab physicist Stan Tozer, scientific advisor on this story. For more information on this measurement technique, please visit the High Pressure in DC Fields page.
By Kristen Coyne
Last modified on 02 December 2022

IMAGES
VIDEO